Adverse
cutaneous drug reactions S ES E C T I O N 2
Introduction
A drug may be defined as a chemical substance, administered for the
investigation, prevention or treatment of diseases or symptoms, real or
imagined. An adverse drug reaction (ADR) may be defined as an undesirable
clinical manifestation resulting from administration of a particular drug. The
skin is often the first place where ADRs are noted when we call adverse cutaneous
drug reactions (ACDRs).
Cutaneous
reactions to drugs occur in up to 8% of hospitalized patients. Up to 5% of patients treated with antibiotics and aromatic
anticonvulsants may develop a cutaneous eruption. Approximately 2% of all
drug-induced skin reactions are considered “serious” according to the following
World Health Organization (WHO) definition: “if it results in death, requires
hospitalization or prolongation of existing hospital stay, results in
persistent or significant disability/incapacity, or is life-threatening”. Toxic
epidermal necrolysis (TEN) and drug reaction with eosinophilia and systemic
symptoms (DRESS; also referred to as drug-induced hypersensitivity syndrome
[DIHS]) are examples of such “serious reactions”. Roujeau and
Stern estimated that 1 of every 1000 hospitalized patients has a serious
cutaneous drug reaction. Prompt identification of severe cutaneous adverse
reactions (SCARs) is an important goal, followed by discontinuation of the most
likely offending drug(s) and thereby decreasing morbidity
Risk of adverse drug reactions among different patient
groups
Certain patient groups are at increased risk of developing an ADR.
Age: The incidence of adverse reactions increases with patient age, the elderly
have a significantly higher incidence of ADRs, related to decreased organ
reserve capacity, altered pharmacokinetics and pharmacodynamics, and
polypharmacy.
Sex: Women are more likely than men to develop ADRs.
Immunosuppressed: patients are most frequently
affected, such as those with connective tissue diseases, including systemic
lupus erythematosus (SLE), AIDS patients and lymphoma.
Pathogenesis
Drug-induced skin reactions are
mediated by either nonimmunologic or immunologic
mechanisms. About 80% of drug reactions are nonimmunologic which are
predictable, usually dose-dependent, related to the known pharmacological
actions of the drug and occur in otherwise normal individuals. Side effects are
unavoidable at the regular prescribed dose.
Immunologic reactions are unpredictable, dose-independent, are not related
to the pharmacological action of the drug and may have a basis in genetic
variation in drug bio activation and drug or metabolite detoxification or
clearance. In immunologic reactions, drugs or their metabolites
act as haptens, inducing a specific cell-mediated or humoral response.
|
MECHANISMS OF CUTANEOUS DRUG-INDUCED REACTIONS |
|
|
Immunologic mechanism (unpredictable) |
·
IgE-dependent drug reactions ·
Cytotoxic drug-induced reactions ·
Immune complex-dependent drug reactions ·
Cell-mediated reactions |
|
Non-immunologic mechanisms (sometimes predictable) |
·
Overdose ·
Pharmacologic side effects ·
Cumulative toxicity ·
Delayed toxicity ·
Drug–drug interactions ·
Alterations in metabolism ·
Exacerbation of disease |
|
Idiosyncratic with a possible immunologic mechanism
(unpredictable) |
·
DRESS ·
SJS/TEN ·
Drug reactions in the setting of HIV
infection ·
Drug-induced lupus(LE) . |
Immunologically Mediated Drug
reactions
·
IgE-dependent drug reactions (formerly
type I, Gell–Coombs classification): urticaria, angioedema, and anaphylaxis.
·
Cytotoxic drug-induced reactions (antibody
against a fixed antigen; formerly type II): petechiae secondary to drug-induced
thrombocytopenia.
·
Immune complex-dependent drug reactions (formerly
type III): vasculitis, serum sickness, and certain types of urticaria.
·
Delayed-type, cell-mediated drug reactions (activation
of CD4+ and CD8+ T cells;
formerly type IV): exanthematous, fixed, and lichenoid drug eruptions, as well
as Stevens–Johnson syndrome (SJS) and TEN. This group has been further
subdivided into the following:
1. Th1
immune reaction (IVa): monocytes are preferentially recruited and activated by
IFN-γ, leading to CD8+ T-cell
activation and a proinflammatory response (TNF, IL-12)
2. Th2
immune reaction (IVb): eosinophils are preferentially recruited and activated
in part by IL-4, -5, -13 and eotaxin, as in DRESS
3. Cytotoxic
immune reaction (IVc): involves CD4+ and CD8+ T cells with release of perforin and granzyme
B and/or Fas–FasL interactions, as in SJS/TEN
4. Neutrophil
and T-cell-based immune reaction (IVd): mediated via chemokines (e.g. CXCL8)
and cytokines (e.g. GM-CSF), as in acute generalized exanthematous pustulosis
(AGEP)
Several
immunologic mechanisms have been proposed to explain SCARs including:
(1) The hapten/pro-hapten concept – the drug or its
metabolite covalently binds to an endogenous peptide and the resultant hapten
is recognized by a highly restricted major histocompatibility complex (MHC);
(2) The pharmaco immune reaction (“p-i concept”) – drugs
induce formation of HLA–drug complexes that can directly activate T-cell immune
responses without the need for a specific peptide ligand; and
(3) Direct HLA–drug interactions – certain drugs such
as carbamazepine, abacavir and sulfamethoxazole bind non-covalently within the
peptide groove of a specific HLA and modify the antigen-binding cleft, thereby
altering the endogenous peptide repertoire.
Genetic
factors
Specific
HLA alleles have emerged as important genetic risk factors for SCARs,
especially SJS/TEN and DRESS. Given the strong associations between HLA-B*1502
and carbamazepine-triggered SJS/TEN in Asians and between HLA-B*5701 and
abacavir-triggered DRESS, pretreatment genetic testing is now recommended.
Non-immunologic Mechanisms
Overdose
The
clinical manifestations of a drug overdose are predictable and represent an
exaggeration of the medication’s pharmacologic actions. It may occur as a
consequence of a prescribing error, deliberate excess by the patient, or
altered absorption, metabolism or excretion. An example of the latter is
methotrexate toxicity in elderly patients with reduced renal function.
Pharmacologic
side effects
These reactions include
undesirable or toxic effects that cannot be separated from the desired
pharmacologic actions of the drug. An example would be alopecia and mucositis
due to chemotherapeutic agents that target more rapidly dividing cells.
Cumulative
toxicity
Prolonged exposure to a
medication or its metabolites may lead to cumulative toxicity. For example,
methotrexate can lead to hepatic fibrosis and accumulation of minocycline or
amiodarone within the skin can lead to cutaneous discoloration.
Delayed
toxicity
This corresponds to a toxic,
dose-dependent effect that occurs months to years after the discontinuation of
a medication. Examples include squamous cell carcinomas and palmoplantar
keratoses following exposure to arsenic and acute leukemia due to alkylating
agents.
Drug–drug
interactions
Interactions
between two or more drugs administered simultaneously may occur at several
different steps: (1) intestinal drug interactions; (2) displacement from
binding proteins or receptor sites; (3) enzyme stimulation or inhibition; and
(4) altered drug excretion. Examples of each include the interactions between
tetracycline and calcium, methotrexate and sulfonamides, cyclosporine and
azoles, and methotrexate and probenecid.
Alterations
in metabolism
Drugs may induce cutaneous
changes by their effects on the nutritional or metabolic status of the patient.
Bexarotene may induce severe hypertriglyceridemia and eruptive xanthomas, while
isoniazid may be associated with pellagra-like changes.
Exacerbation
of disease
A variety of drugs can exacerbate
pre-existing dermatologic diseases, such as androgens in patients with acne
vulgaris or lithium and interferon in patients with psoriasis.
Idiosyncratic with a Possible Immunologic Mechanism
The pathophysiology of
drug-induced skin reactions such as exanthematous drug eruptions, DRESS, AGEP
and TEN, as well as the increased susceptibility of HIV-infected patients, may
be partially explained by an interplay between immune mechanisms and genetic
predisposition (e.g. slow versus rapid acetylators).
Diagnostic Features
Drug
eruptions, suspected and unsuspected, frequently lead to a dermatologic
consultation, and it is often (although not always) possible to categorize a
drug as having a high, medium or low probability of being the culprit. A
logical approach begins with an accurate description of the skin lesions and
their distribution, in addition to associated signs and symptoms. Data regarding
all the drugs taken by the patient, including prescription,
non-prescription/over-the-counter and complementary or alternative treatments,
as well as the dates of administration and doses need to be collected. The
chronology of drug administration is of paramount importance. The time between
initiation of the drug and the onset of the skin eruption is a key element in
identifying the offending drug, as most immunologically mediated reactions
occur 8 to 21 days after initiation of a new medication.
|
LOGICAL APPROACH TO DETERMINE THE CAUSE OF A DRUG ERUPTION |
|
|
Drug
responsibility assessment |
|
|
Clinical characteristics |
·
Type of primary lesion (e.g. urticaria,
erythematous papule, pustule, purpuric papule, vesicle, or bulla) ·
Distribution and number of lesions ·
Mucous membrane involvement, facial edema ·
Associated signs and symptoms: fever,
pruritus, lymph node enlargement, visceral involvement |
|
Chronological factors |
·
Document all drugs to which the patient has
been exposed (including OTC and complementary) and the dates of
administration ·
Date of eruption ·
Time interval between drug introduction (or
reintroduction) and skin eruption ·
Response to removal of the suspected agent ·
Consider excipients (e.g. soybean oil) ·
Response to rechallenge (often inadvertent) |
Evolution after drug withdrawal
may be helpful, as the cutaneous eruption usually clears when the suspected
drug is discontinued. However, this assessment may prove difficult in the case
of drugs with a long half-life or “persistent” drug reactions such as lichenoid
and photo allergic eruptions or drug-induced pemphigus foliaceus and subacute
cutaneous lupus erythematosus.
The suspect drug should be
withdrawn as soon as possible. The usual practice is to discontinue all drugs
that are non-essential. However, in some instances, it is necessary to weigh
the risks versus the benefits of each drug and to determine if a
similar-acting, but non-cross-reactive, drug is available as a substitute.
In the
process of identifying the responsible drug, access to drug databases is very
helpful. However, new or unusual drug reactions may not be identified.
Moreover, the drug most frequently associated with adverse reactions may be
innocent in a particular patient, and the physician dealing with a suspected
drug reaction must remain open-minded.
Rechallenge
carries the risk of inducing a more severe reaction, thus limiting its use for
both ethical and medico-legal reasons. Furthermore, the recurrence rate is not
100% with rechallenge (e.g. there are refractory periods) and a negative result
may give an erroneous sense of security. Even with these limitations, in
patients with fixed drug eruptions, topical provocation or rechallenge may
prove helpful.
Findings indicating possible
life-threatening ACDR
• Skin pain.
• Confluent erythema.
• Facial edema or
central facial involvement
• Palmar/plantar painful
erythema.
• Epidermal
detachment and blisters.
• Positive Nikolsky
sign.
• Mucous membrane
erosion.
• Urticaria.
• Swelling of the
tongue.
• High fever (temperature
> 40 degree C).
• Enlarged lymph
nodes.
• Arthralgia.
• Shortness of
breath, wheezing, and hypotension.
• Palpable purpura.
• Skin necrosis.
CLINICAL TYPES OF ADVERSE DRUG REACTIONS
Drug eruptions can mimic all the morphologic form in dermatology and must
be suspected when an eruption appear suddenly.
Clinically cutaneous drug reactions commonly present as:
EXANTHEMATOUS,
URTICARIA/ANGIOEDEMA, PUSTULAR or BLISTERING
Exanthematous
reactions include maculopapular rashes and drug hypersensitivity syndrome.
Urticarial reactions include urticaria, angioedema, and serum sickness-like
reactions. Blistering reactions include fixed drug eruptions, Stevens-Johnson’s
syndrome, and toxic epidermal necrolysis. Pustular eruptions include acneiform
drug reactions and acute generalized exanthematous pustulosis.
Drug‐induced
exanthem
Introduction
Exanthematic
eruptions can be caused by a variety of drugs and resemble in appearance the
classical rash of a viral infection, for which the paradigm is the morbilliform
rash associated with measles. Typically, exanthematic drug reactions do not
show the systemic involvement, such as fever, that would characterize a viral
infection.
It is most common type of cutaneous drug reactions accounting for 90% of
skin reactions.
Etiology
Any drugs can induce an exanthematous drug reaction in approx. 1% of
patients, but few drugs can affect >3% of patients: aminopenicillins,
sulphonamides, cephalosporin, aromatic anticonvulsants. non‐steroidal
anti‐inflammatory drugs (NSAIDs) and allopurinol.
Certain
viral infections are also known to increase the incidence of drug reactions.
Depending upon the series, the frequency of aminopenicillin-induced
exanthematous eruptions in patients with infectious mononucleosis ranges from
33% to 100%. One theory is that reactive drug metabolites disturb the balance
between cytotoxic and regulatory immune responses, leading to a cytotoxic
reaction that targets virally infected keratinocytes.
Pathophysiology
The primary underlying pathomechanisms are most likely
immunologic, complex, and cell-mediated. Several mechanisms have been proposed
(see above) in which the drug or drug-peptide hapten presented by dendritic
cells to T lymphocytes can either bind covalently or non-covalently to MHC
molecules. CD4+ and CD8+ T cells that strongly express perforin and
granzyme are then recruited and their cytotoxic activity leads to death of
keratinocytes.
Clinical features
The eruption, macules and/or papules classically begins 1-3 weeks after the
start of a new medication, on the trunk and spreads peripherally in a bilateral
and symmetric fashion to the extremities accompanied by pruritus, intertriginous
areas may be a favored site but the face is typically spared. In children, it may be limited to the face
and extremities. In time, lesions become confluent forming large patches,
polycyclic/gyrate erythema, reticular eruptions, or sheet-like erythema. It is typically confluent on the trunk, and discrete on the extremities. Palmar and plantar
lesions may occur, and sometimes the eruption is generalized. In individuals
with thrombocytopenia, exanthematous eruptions can mimic vasculitis because of intralesional
hemorrhage. The eruption is typically more
polymorphous than a viral exanthem. Sometimes, due to dependency, the lesions
on the distal lower extremities become petechial or purpuric. Mucous membranes
are usually spared but pruritus and a low-grade fever are often present. There
may also be annular plaques or atypical “target” lesions, leading to a
misdiagnosis of erythema multiforme. There may be relative sparing of
pressure areas. It can even occur 1-2 days after the drug has been
discontinued and within 2-3 days after re-administration of drug in previously
sensitized patient.
Differential diagnosis
Viral infection is
the most important differential diagnosis. It is useful in differentiating
exanthematic drug eruptions from viral exanthems to remember that viral rashes
tend to start on the face and acral sites with subsequent progression to
involve the trunk, and are more often accompanied by fever, sore throat,
gastrointestinal symptoms, conjunctivitis, cough and insomnia. Enanthems, which
involve the mucous membranes, are more commonly associated with an infectious
etiology. Peripheral blood eosinophilia and a polymorphous appearance point
to a drug eruption, and in the absence of definitive evidence, drug eruptions
are favored in adults whereas viral exanthems are favored in children.
Disease course and prognosis
The illness follows a
benign course, and resolves without complications
and/or sequelae following cessation of the offending drug. Once the drug is discontinued, the eruption disappears spontaneously after
1-2 weeks with a change in color from bright red to dark red, which may be
followed by desquamation,
sometimes with post‐inflammatory hyperpigmentation. However, for 1 to 3 days immediately following
discontinuation of the responsible medication, an increase in extent and
intensity may be observed. If the administration of the drug is
continued, an exfoliative dermatitis may develop, although occasionally the
eruption subsides despite continuation of the medication. Morbilliform drug
eruptions usually, but not always, recur on rechallenge.
Of more concern, a morbilliform eruption may be the initial presentation of
a more serious eruption, i.e., SJS, TEN, or drug hypersensitivity syndrome. Signs
and symptoms that point to the possibility of a more severe drug-induced
eruption include edema of the face, pustules, vesicles, dusky or painful
lesions, skin fragility, mucous membrane involvement, and marked peripheral
blood eosinophilia.
Pathology
Histology
is generally non‐specific i.e. a
mild superficial perivascular and interstitial lymphocytic infiltrate that may
contain eosinophils (up to 70% of cases) in addition to interface changes.
Investigations
Blood tests to
exclude organ dysfunction and hematological abnormalities associated with DRESS
are important.
Treatment
Cessation
of the culprit drug is essential. Generally, symptomatic treatment only is
required; most cases benefit from emollients. Approximately 50% of
exanthematous eruptions are pruritic and intermediate potency topical
corticosteroids may be useful for these cases.
Resolution
of drug‐induced exanthems is faster than for
infectious exanthems.
Drug- induced acute urticaria,
angioedema, and anaphylaxis
Salient features
• Drug-Induced
urticaria and angioedema occur, caused by a variety of mechanisms and are characterized
clinically by transient wheals and angioedema causing extensive tissue swelling
with involvement of deep dermal and subcutaneous tissues. Angioedema is often
pronounced on the face or mucous membranes (tongue).
• In
some cases, cutaneous urticaria/angioedema is associated with systemic
anaphylaxis, which is manifested by respiratory distress, vascular collapse,
and/or shock.
Time from initial Drug Exposure
to Appearance of Urticaria
• IgE-Mediated:
Initial sensitization, usually 7 to 14 days. In previously sensitized
individuals, usually within minutes or hours.
•
Immune Complex-Mediated: Initial sensitization, usually 7 to 10 days, but as
long as 28 days; in previously sensitized individuals, 12 to 36 h.
• Analgesics/Anti-Inflammatory
Drugs: 20 to 30 min (up to 4 h).
Prior Drug Exposure
Radiographic
Contrast Media: 25 to 35% probability of repeat reaction in individuals with
history of prior reaction to contrast media.
Skin symptoms
Pruritus,
burning of palms and soles, airway edema with breathing difficulties
Constitutional symptoms
IgE-mediated:
Flushing, sudden fatigue, yawning, headache, weakness, and dizziness; numbness
of tongue, sneezing, bronchospasm, substernal pressure, and palpitations;
nausea, vomiting, crampy abdominal pain, diarrhea, and possibly arthralgia.
Introduction
Urticaria
and angioedema are physical signs characterized by
short-lived swellings of the skin and mucosa due to plasma leakage. Anaphylaxis is a
constellation of clinical findings which describes respiratory and
cardiovascular compromise (bronchoconstriction and hypotension) in a life‐threatening
manner.
Drug‐induced
urticaria can be seen in isolation or in association with anaphylaxis and
angioedema. Aspirin and NSAIDs are the most frequently implicated drugs causing
urticaria and β‐lactams antibiotics and NSAIDs are the most
frequently implicated drugs causing anaphylaxis.
Urticaria develops in about 1% of patients receiving blood transfusions; it
may also follow alcohol consumption, intraarticular methylprednisolone and even
cetirizine.
Epidemiology
Urticaria is the second most common type of ACDR after exanthematous
eruption.
Pathophysiology
In urticaria, there is vasodilation and transient edema within the
superficial dermis whereas in angioedema, the edema is present in deep dermal,
subcutaneous, and submucosal tissues. The mechanisms
underlying drug‐induced urticaria, angioedema (deeper in
skin) and anaphylaxis (systemic circulation) are identical. Classically, these
reactions are mediated by the presence of drug‐specific IgE. On
exposure to the drug, cross‐linking of IgE on the
surface of mast cells (and possibly basophils) is followed by inflammatory
mediator release (including histamine), which induces vasodilation, neuronal
activation and smooth muscle contraction. Clinically,
“anaphylactoid” reactions may mimic IgE-induced histamine release, but are
secondary to a non-immunologic liberation of histamine and/or other mediators
of inflammation. Cyclo‐oxygenase inhibitors,
such as aspirin and indometacin, may also cause urticaria or angioedema by
pharmacological mechanisms. Other drugs, such as radiocontrast media, local anesthetics and dextrans (in plasma expanders) may release mast cell mediators
directly. Angiotensin‐converting enzyme (ACE) inhibitors can cause
angioedema which is bradykinin mediated rather than histamine dependent and
will therefore not respond to antihistamines.
Clinical features
Urticaria,
angioedema and anaphylaxis arise within 24–36 h of drug ingestion at the
first occasion. On re‐challenge lesions may develop within minutes.
Anaphylaxis usually develops on second exposure to a drug. Anaphylaxis and
anaphylactoid reactions usually develop within minutes to hours (the vast majority
within the first hour) of drug administration.
Urticaria
Urticaria
presents as transient, often pruritic, erythematous and edematous papules and
plaques that may appear anywhere on the body, including the palms, soles, and
scalp. Lesions can vary significantly in size and number and may assume a
figurate configuration. The primary effector cell is the cutaneous mast cell
which releases histamine and other inflammatory mediators.
Although drugs are thought to be
responsible for <10% of all cases of urticaria, they are more often
associated with acute rather than chronic urticaria. That said, patients with
chronic urticaria should avoid acetylsalicylic acid (ASA; aspirin), as well as
other NSAIDs, as they can lead to an exacerbation.
In IgE-mediated urticaria,
lesions typically appear within minutes to less than an hour after drug
administration, especially when there has been prior sensitization. Both
immunologic assays, such as radioallergosorbent tests (RAST) that detect
specific IgE antibodies and skin tests (prick tests) can prove useful in
confirming the diagnosis. However, the number of drugs for which there are
commercially available assays is limited, consisting primarily of penicillin,
aminopenicillin, cephalosporin, and insulin. Of course, prick tests should be
performed under appropriate medical supervision due to the risk of an
anaphylactic reaction. It should also be noted that in some series, only 10–20%
of patients who reported a history of penicillin allergy were truly allergic
when assessed by skin testing.
Drugs
that most frequently produce immunologically-based urticaria are antibiotics,
especially penicillins and cephalosporins, and less often, sulfonamides and
minocycline. As the use of monoclonal antibodies for neoplastic and inflammatory
diseases increases, so will cases of urticaria (and vasculitis; see below) due
to these exogenous proteins.
In
anaphylactoid reactions, vasodilation results from the liberation of large
amounts of histamine, bradykinin, and/or leukotrienes. Acetylsalicylic acid is
the classic example of a drug that induces an anaphylactoid reaction and it
does so via cyclooxygenase inhibition and subsequent accumulation of
leukotrienes. The majority of urticarial reactions to radiocontrast media are
also non-immunologic, as are many, but not all, reactions to NSAIDs (e.g.
ibuprofen, naproxen). Allergic reactions to latex in gloves or medical devices
can induce local or generalized urticaria, especially in the case of direct
contact with mucosal surfaces.
|
ADVERSE REACTIONS TO RADIOCONTRAST MEDIA |
|
Iodinated |
|
1.
Immediate (<1 hour) · Urticaria
(usually non-immunologic), angioedema · Anaphylactoid
reaction 2.
3.
Delayed (1 hour to 1 week) · Morbilliform
eruption · Acute
generalized exanthematous pustulosis (AGEP) · Symmetrical
drug-related intertriginous and flexural exanthema (SDRIFE) · Iododerma
(also >1 week) |
|
Gadolinium |
|
>Urticaria, erythema (rare) >Nephrogenic systemic fibrosis (in the setting of renal
insufficiency) |
Angioedema
Angioedema
is a reflection of transient edema of the deep dermal, subcutaneous and
submucosal tissues. It is associated with urticaria in 50% of cases and may be
complicated by life-threatening anaphylaxis. Angioedema occurs in 1 to 2 per
1000 new users of angiotensin-converting enzyme (ACE) inhibitors and is due to
an accumulation of bradykinin. The most severe cases of angioedema may start
within a few minutes after drug administration. However, in the case of ACE
inhibitor-induced angioedema, lesions may appear from 1 day to several years
after starting the drug; most appear within the first year. Women are at
increased risk for developing ACE inhibitor-induced angioedema.
The most common clinical
presentation is an acute, asymmetric, pale or pink, subcutaneous swelling
involving the face. Involvement of the oropharynx, larynx, and epiglottis can
lead to impaired swallowing and stridor. Occasionally, in drug-induced
angioedema, there is edema of the intestinal wall with abdominal pain, nausea,
vomiting, and diarrhea.
The major
drugs implicated in angioedema, besides penicillins and ACE inhibitors, are
NSAIDs, radiographic contrast media and, more recently, monoclonal antibodies.
Although angiotensin II receptor antagonists do not increase levels of bradykinin,
they are also associated with angioedema, albeit less frequently. It is
important to note that drug-induced angioedema may actually represent an
unmasking of another cause for angioedema, e.g. acquired C1 inhibitor
deficiency due to autoimmune or lymphoproliferative disorders.
Anaphylaxis
Anaphylaxis
consists of an acute life-threatening reaction that occurs within minutes of
drug administration, usually parenteral. It occurs in about 1 per 5000
exposures to penicillin. Intravenous
administration is associated with more severe reactions and rapid progression.
Etiopathogenesis
Anaphylaxis is caused by activation of cells of the
immune system, primarily mast cells in the skin and mucous membranes and
basophil granulocytes in the blood. This activation can take place either via
immunoglobulin E antibodies or by direct release of mediators (non-immunological
anaphylaxis, pseudo-allergy). Risk factors for severe anaphylaxis include
underlying diseases such as mastocytosis, the use of β-blockers or angiotensin-converting
enzyme inhibitors. In certain patients, anaphylactic symptoms occur only after
the combined action of various stimuli, such as physical exertion, mental or
emotional stress, acute infection or simultaneous exposure to irritants (e.g.,
alcohol) or additional allergens. For this phenomenon, it is often used the
term summation or augmentation anaphylaxis and
consider it much more common than generally suspected.
The most frequent triggers of anaphylactic reactions are
the following:
·
Drugs (all forms of application)
·
Food products
·
Insect venoms
·
(in Childhood foods are the most frequent elicitors)
Other triggers are:
·
Aeroallergens
·
Contact urticariogens
·
Seminal fluid
·
Occupational substances (e.g., latex)
·
Cold, heat, UV radiation
·
Echinococcal cysts
·
Additives
·
Summation factors, including exercise, infection, stress,
concomitant exposure to other allergens, medication: β blockers, NSAIDs
·
Idiopathic
·
Underlying diseases: C1 inhibitor deficiency, systemic
mastocytosis
The most
frequently incriminated drugs are antibiotics, in particular the
penicillins/aminopenicillins but also cephalosporins and quinolones; additional
causes are muscle relaxants (e.g. suxamethonium), acetaminophen, and
gadolinium-based contrast media. Anaphylaxis can also be seen following
exposure to latex while anaphylactoid reactions are usually seen with NSAIDs
and radiocontrast media. Rarely, anaphylaxis occurs following cutaneous
injections (e.g. local anesthetics) or topical applications of medications
(e.g. bacitracin, chlorhexidine).
Clinical Features
Clinically, anaphylaxis manifests as a syndrome, with
various organ manifestations that can occur one after the other, simultaneously
or individually. The skin is particularly often affected, with flushing,
itching, hives or angioedema.
The gastrointestinal tract can be involved in the form of
nausea, abdominal pain, vomiting, and diarrhea. Rhinitis, hoarseness, laryngeal
swelling, shortness of breath with bronchoconstriction, asthma, and even
respiratory arrest occur in the respiratory tract.
In the cardiovascular system, tachycardia and
fluctuations in blood pressure (often a drop, but sometimes an acute rise in
blood pressure), as well as cardiac arrhythmia, are frequently observed, which
can lead to circulatory shock and circulatory arrest.
Subjective symptoms (formerly called “prodromes”) include
paresthesia of the palms or soles, itch in the anogenital area, metallic or
fishy taste, anxiety, sweating, and disorientation.
According to the intensity of the symptoms,
classification into degrees of severity of I-IV has proven successful. Not all
symptoms have to be present.
|
Degree |
Skin and subjective general
symptoms |
Abdomen |
Respiratory tract |
Cardiovascular system |
|
I |
Itching Flush Urticaria Angioedema |
– |
|
|
|
II |
(Not
obligatory) |
Nausea Cramps |
Rhinorrhea Hoarseness Dyspnea |
Tachycardia Hypotension
Arrhythmia |
|
III |
” |
Vomiting Defecation |
Laryngeal edema Bronchospasm Cyanosis |
Shock,
unconsciousness |
|
IV |
” |
” |
Apnea |
Circulatory
arrest |
Disease course and prognosis
On withdrawal of the
offending culprit, clinical improvement in drug‐induced urticaria and
angioedema occurs within 24–48 h. It is important to recognize that after
an initial improvement from anaphylaxis, late‐phase reactions may
arise 5–6 h afterwards. Deaths from drug‐induced anaphylaxis
are uncommon (<2%); the major predisposing risk factor for poor outcome is
coexistent severe asthma.
Treatment
For
evolving urticaria with or without angioedema or anaphylaxis, the key principal
management step is to stop the offending drug and disease resolution occurs
quickly.
Acute measures for the therapy of anaphylactic reactions
follow guidelines and depend on the severity of the symptoms. If the reaction
only extends to the skin and the breathing and circulation are stable, initial
therapy can be done with antihistamines. In all patients, intravenous access
has to be established. If circulation or breathing is affected, adrenaline
therapy is required. Adrenaline autoinjectors for intramuscular self-medication
(e.g. EpiPen) is commercially available, which differ from one another slightly
in handling. Initially, 300–500 μg is administered intramuscularly to adults.
In severe therapy-resistant reactions, 1:10 diluted adrenaline should be
applied slowly intravenously or by infusion of 1:100 dilutions under continuous
pulse and blood pressure control. In the case of predominant respiratory
symptoms, β2-adrenergic receptor agonists or adrenaline are given by
inhalation. In hypotension, volume therapy (up to 1–3 l for adults initially)
is of crucial importance. Of note, patients taking β-blockers
may have a limited response to epinephrine. In cases of insect sting‐related
and food‐induced anaphylaxis, the syndrome evolves
more slowly.
The use of glucocorticoids in anaphylactic shock is
controversially discussed, as glucocorticoids require up to 60 min to take its effect.
However, their action may be needed for suppressing the late phase reaction
after 6–12 h. In the event of respiratory and/or circulatory arrest, the acute
measure of cardiopulmonary resuscitation (CPR) must be applied.
As most anaphylactic reactions are accompanied by skin
symptoms and skin symptoms are often the first visible symptoms of incipient
anaphylaxis, the dermatologist is at the forefront of managing this most severe
and life-threatening form of immediate allergic reaction.
Patients should avoid all NSAIDs if possible. Paracetamol, ibuprofen, and
tramadol have a low risk of urticaria and can be substituted for the other
NSAIDs.
Drug‐induced
serum sickness‐like
reaction
Introduction
Drug‐induced
serum sickness‐like reactions (SSLR) are characterized by a
clinical triad of fever, rash and arthralgia/arthritis.
SSLR is a drug
reaction pattern, so named because of its similarity in clinical presentation
to serum sickness. Classical serum sickness is a type III hypersensitivity
reaction to foreign proteins (e.g. antithymocyte
globulin, tositumomab, infliximab) resulting in the deposition of immune
complexes in small blood vessels of various organs such as the skin, joints and
other systems. In distinction, SSLR is typically due to medications and despite
the clinical similarity, there is no circulating immune complexes, no vasculitis, no renal lesions with normal complement
level.
Incidence and prevalence
Cefaclor
is the most common reported trigger, occurring at 1 per 3000 cefaclor
prescriptions compared to 1 per 120 000 in the case of amoxicillin. Other drugs associated with serum sickness-like reactions
are penicillins, NSAIDs, bupropion, phenytoin, sulfonamides, minocycline, and
propranolol.
Age
SSLR is more commonly
reported in children and this may reflect the relatively frequent use of high‐risk
medications such as cefaclor in the young.
Pathophysiology
The
pathophysiology of SSLR is not well studied. Biotransformation of the parent
drug to reactive metabolites is essential and inherited defects in the
metabolism of these reactive intermediates may be a predisposing factor.
Clinical features
History
The
typical median latency from drug initiation to onset of rash ranges is 7 days
with a range from 1 to 13 days.
Presentation
The
primary lesions of SSLR are urticarial weals. Some lesions may have purpuric or
dusky centers and may be mistaken for erythema multiforme. Facial and
periorbital edema may coexist. No mucosal membranes are involved.
Joints of the hands
and feet are also often affected; associated symptoms include arthralgia,
swelling, warmth and decreased range of movement. Systemic involvement of the
kidneys and liver is rare, in contrast to true serum sickness.
Disease course and prognosis
Cutaneous and
musculoskeletal manifestations resolve on drug withdrawal.
Treatment
Withdrawal of the
culprit drug is essential; the rash and joint symptoms usually resolve within 1
week of withdrawal. Symptomatic treatment such as antihistamines, antipyretics
and systemic corticosteroids are required.
Drug eruption, fixed
Introduction
Fixed drug eruption
(FDE) is a cutaneous adverse drug reaction characterized by recurrent well‐defined
lesions occurring in the same sites each time the offending drug is taken. It
has short latency and is benign in nature. The drugs
most frequently associated with FDE include antibiotics (Co‐trimoxazole, tetracyclines > β-lactams,
fluoroquinolones, macrolides), NSAIDs, paracetamol (acetaminophen), aspirin, barbiturates, dapsone, proton
pump inhibitors, and azole antifungal drugs.
Age
FDE has been reported
in all ages. However, it is more common in adults, particularly in the range of
40–80 year olds.
Pathophysiology
FDE
is a form of classical delayed‐type hypersensitivity
reaction and skin resident T cells are believed to be the key mediators in
eliciting FDE. Long after clinical resolution, ‘resting’ FDE lesions contain
CD8+ T cells with an effector/memory phenotype. These cells are located at the
dermal–epidermal junction and remain quiescent until drug re‐challenge.
On re‐exposure to the drug, there is activation and
expansion of these CD8+ lymphocytes with the release of interferon (IFN) ‐γ
and cytotoxic granules resulting in keratinocyte apoptosis.
Pathology
Early
biopsy specimens show an interface dermatitis reaction pattern with vacuolar
degeneration of basal keratinocytes, papillary dermal edema and a perivascular
lymphocytic infiltrate of the upper dermis. There may
be scattered necrotic keratinocytes in the epidermis or extensive epidermal
necrosis. Resolved or healing lesions are characterized by pigment‐laden
macrophages in the upper dermis.
Clinical features
History
Lesions
develop from a few days to two weeks after the initial exposure. With
subsequent exposures, they appear within 24 hours (30 min to
8 h).
Presentation
Typically,
FDE presents as a sharply‐defined, round or oval erythematous and
edematous plaque which evolves to become dusky, violaceous and occasionally may
become bullous and then eroded due to epidermal detachment. Eroded lesions,
especially on genitals or oral mucosa, are quite painful. Lesions are usually
solitary or few in number although multiple lesions may be present or may
develop as a consequence of repeated challenges.
Although FDEs can occur anywhere on the skin surface as well
as the mucous membranes, they are commonly found
on the perioral and periorbital areas, hands and feet, glans penis, and oral
mucosa. The initial acute phase lasts for days to weeks and then heals leaving dark brown with
violet hue post inflammatory hyper pigmentation; pigmentation may be all that is visible between attacks. Upon
re-administration of the causative drug, lesions recur in exactly the same
sites. With each subsequent recurrence, additional sites of involvement may
appear or the number of lesions may remain constant. Typically, the time interval between exposure and development of the
lesions shortens on repeated exposures.
Drug‐specific
clinical patterns have been reported. These include: NSAID‐induced
FDE affecting the genitals and lips; tetracycline‐ and
trimethoprim/sulfamethoxazole‐induced FDE affecting
the genitals.
The presence of numerous lesions is referred to as generalized
bullous FDE. Generalized
bullous FDE (GBFDE) is a form of extensive FDE which may be misdiagnosed as
toxic epidermal necrolysis (TEN). Differentiating features from TEN include:
(i) prior history of similar episodes; (ii) mucosal surfaces are relatively
uninvolved; (iii) the presence of large blisters with intact intervening skin;
and (iv) the absence of multiple purpuric or atypical target macules. In
generalized disease, prognosis correlates with the extent of skin detachment.
Different morphological variants of FDE:
·
Pigmented FDEs
· Nonpigmented FDEs
· Bullous
FDEs
· Multifocal
FDEs
· Erythema
multiforme like FDEs
· Linear
FDEs
· Generalized
bullous/TEN like FDEs
· Wandering
FDEs
Complications and co‐morbidities
Patients with GBFDE
generally lack visceral complications and are thought to have a benign clinical
course. In contrast with SJS/TEN, GBFDE generally has modest or no mucosal
involvement and is not associated with respiratory tract or intestinal
involvement. However, the mortality in GBFDE was not significantly lower that
of SJS/TEN.
Disease course and prognosis
The majority of FDE
is self‐limiting with an excellent prognosis. Post‐inflammatory
hyperpigmentation can be prominent and persist for several months after the
acute episode. In GBFDE, the mortality is approximately 20%. Patients with
GBFDE require the same level of treatment and care as for SJS and TEN.
In some
patients, the responsible drug can be re-administered without inducing an
exacerbation, and there may be a refractory period after the occurrence of a
fixed eruption. Provocation via patch testing in a previously involved site may be useful in determining the
responsible drug (as long as not performed during the refractory period).
Treatment
Treatment involves stopping
the offending drug. For noneroded lesions: Potent topical glucocorticoid
ointment and for eroded lesions: Antimicrobial ointment. For widespread,
generalized, and highly painful mucosal lesions, oral prednisone 1 mg/kg body
weight tapered over a course of 2 weeks. GBFDE should be treated in an
intensive care center with expertise in skin loss syndromes, or in a burns
unit.
Drug
reaction with eosinophilia and systemic symptoms (DRESS)
Introduction
DRESS
is an idiosyncratic, unusual, potentially
life-threatening, multi-organ adverse drug
reaction that begins acutely in the first 2 months after initiation of
the drug. It is characterized by cutaneous features, namely a rash, and
systemic involvement. The latter includes hematological disturbance, with
eosinophilia being the most consistent finding. Leucocytosis, lymphopenia,
lymphocytosis, thrombocytosis and thrombocytopenia are also described. Atypical
lymphocytes are a common finding on blood film in DRESS patients, the presence
of which is used as a component of the diagnostic criteria. Lymphadenopathy is
found in more than 75% of patients, with involvement of two nodal basins
required to meet diagnostic criteria.
Internal manifestations include lymphadenopathy and hepatic
involvement (~80% of patients); rarely, the latter may become life-threatening.
Patients may develop interstitial nephritis, myocarditis, interstitial
pneumonitis, myositis, thyroiditis, and even infiltration of the brain by
eosinophils. The cutaneous and visceral involvement may persist for several
weeks or months after drug withdrawal, and additional sites of involvement
(e.g. cardiac, thyroid) may develop weeks or months later, including following
a taper of corticosteroids.
Management
in the acute phase is centered on the identification and withdrawal of the
culprit drug, and corticosteroid therapy, administered by topical, oral or
intravenous route; the choice being guided by the severity of the disease. In
most patients, the disease is of less than 4 weeks’ duration, with few or no
sequelae. However, a minority of patients enter a chronic phase of disease
characterized by persistence either of the cutaneous features or of the
systemic involvement.
Epidemiology
Incidence
and prevalence
It occurs in approximately 1 in 3000 exposures to
aromatic anticonvulsants (phenytoin, carbamazepine, phenobarbital;
cross-sensitivity among the three drugs is common) and sulphonamide (antimicrobial
agents, dapsone, or sulfasalazine).
Drugs
associated with drug reaction with eosinophilia and systemic symptoms syndrome
(DRESS).
Most
commonly associated drugs are in bold.
|
DRUGS ASSOCIATED WITH DRUG REACTION WITH EOSINOPHILIA AND
SYSTEMIC SYMPTOMS SYNDROME (DRESS) |
|
|
Drug category |
Specific drugs |
|
Anticonvulsants |
Carbamazepine, lamotrigine*,
phenobarbital, phenytoin, oxcarbazepine, zonisamide > valproic
acid |
|
Antimicrobials |
Ampicillin, cefotaxime, dapsone,
ethambutol, isoniazid, linezolid, metronidazole, minocycline, pyrazinamide, quinine, rifampin,
sulfasalazine (salazosulfapyridine), streptomycin, |
|
Antiretrovirals |
Abacavir, nevirapine, zalcitabine |
|
Antidepressants |
Bupropion, fluoxetine |
|
Antihypertensives |
Amlodipine, captopril |
|
NSAIDs |
Celecoxib, ibuprofen |
|
Miscellaneous |
Allopurinol**, azathioprine, imatinib, mexiletine, ranitidine, ziprasidone |
* Especially
when co-administered with valproic acid.
** Full
doses in the setting of renal dysfunction a risk factor.
Age
A
mean age of onset is 48 years.
Pathophysiology
The
two main theories are that of a drug‐specific T‐cell
reaction, and that of viral reactivation.
Some patients have a genetically determined inability to detoxify the toxic
metabolic products of particular drugs due to a defect in normal enzymatic
methods of detoxifying the medications. As a result, this toxic metabolite may
act as a hapten and initiate an immune response. Drug specific activated T
lymphocytes release IL-5 that contributes to eosinophilia, a key feature of
this syndrome.
For example, genetic polymorphisms that affect detoxification of
anticonvulsants and sulfonamides have been identified in patients recovering
from DRESS. For aromatic anticonvulsants, the inability to detoxify toxic arene
oxide metabolites is probably a key factor and would provide an explanation for
the cross-reactivity between phenytoin, carbamazepine, and phenobarbital which
has been well documented, both in vivo and in vitro. Slow N-acetylation of sulfonamide and
increased susceptibility of leukocytes to toxic hydroxylamine metabolites are
associated with a higher risk of hypersensitivity syndrome.
Immune mechanisms have also been implicated based upon several
observations including the requirement for sensitization, positive skin tests
for the culprit drug in some patients, and a shorter time-to-onset upon
rechallenge. Notably, distinct HLA alleles have been associated with a
significantly increased risk of developing drug-specific DRESS.
In addition, IL-5 plays a role in the generation of eosinophilia and
drug-specific T cells, activated in the skin and internal organs, serve to
mediate the disorder.
Herpes
virus reactivation occurs in DRESS. The implicated viruses have included HHV‐6,
CMV, Epstein–Barr Virus (EBV) and HHV‐7. Virus reactivation
appears to occur in a sequential fashion, with HHV‐6
and EBV being detected earlier in the course of the disease, followed by HHV‐7
and CMV. It has been postulated that a drug‐induced immunosuppressed
state, characterized by hypo gammaglobulinaemia, facilitates the initial
reactivation of latent herpes virus. The sequential nature of viral
reactivation suggests a correlation with the clinical phases of DRESS. Rash and
fever are often the first presenting features, followed by lymphadenopathy and
internal organ dysfunction. Some authors have hypothesized that it is the
fluctuation of viral loads that gives rise to these ‘waves’ of disease in
DRESS. It has also been asserted that the persistence of viral reactivation
explains the so‐called ‘chronic’ phase of DRESS experienced
by some patients.
Pathology
Histopathologically, various inflammatory patterns can be seen,
including eczematous, interface dermatitis, AGEP-like, and erythema multiforme-like.
Walsh et al. found a correlation between the
presence of EM‐like changes histopathologically and more
severe liver dysfunction, and suggested that such features may be predictive of
a higher mortality.
Clinical
features
History
Between
2 and 6 weeks, may have elapsed between ingestion of the culprit drug and the
onset of symptoms i.e. later than most other
immunologically mediated skin reactions. With re-exposure, there can be a
shorter time to onset.
Since a familial tendency has been reported, family members of affected
patients should be warned. Familial cases of DRESS to carbamazepine, linked to HLA‐A3101,
have been described.
Presentation
DRESS
is, by definition, characterized by a rash suspected to be drug induced,
accompanied by a fever, lymphadenopathy and systemic involvement, the latter
referring to derangement of the function of at least one organ system, and
hematological abnormalities.
A
clinical classification system for the cutaneous findings in DRESS has been
proposed. The most common variant appears to be the urticated papular exanthem.
This consists of widespread papules and plaques, often accompanied by
cutaneous edema. A morbilliform eruption has been noted in a smaller proportion
of patients, consisting of a pinkish macular rash resembling measles.
Erythroderma may occasionally be the presenting cutaneous feature,
characterized by a widespread exfoliative erythema. A number of patients with
DRESS may present with erythema multiforme‐like features in the
skin, developing dusky or purpuric atypical targets not necessarily confined
to acral sites and may be associated with a more severe systemic phenotype.
Less common manifestations include vesicles, follicular or
non-follicular pustules (~20% of patients), and purpuric lesions on legs.
An
important clinical finding in the majority of patients with DRESS is head and
neck edema. This is often most noticeable by looking at the ears. The face may
be uniformly swollen, or have a more leonine appearance.
The
lesions are distributed symmetrically and almost always occur on the trunk and
extremities. Lesions may become confluent and generalized.
Although
frank mucous membrane involvement is a rarity (and indeed its presence should
call the diagnosis of DRESS into question), cheilitis is a common finding.
Clinical
examination reveals lymphadenopathy in at least two sites in the majority of
patients. One set of clinical criteria require the nodes to be at least
2 cm in diameter to be considered clinically significant.
Regarding
hematological abnormalities, the most common seen is that of eosinophilia.
Pancytopenia is seen in some cases, and is a negative predictive factor in
terms of outcome. A pronounced lymphocytosis may be seen, with levels rising
to >20 × 109 leukocytes/L. It is imperative in all cases for a blood film to
be examined for the presence of atypical lymphocytes, which are frequently
present in DRESS. Leukopenia, lymphopenia (possibly
virally induced) and thrombocytopenia have been noted.
The
liver is the most common viscera to be involved. Although any drug has the potential to cause
liver dysfunction in the context of DRESS, phenytoin, minocycline and dapsone
is the most common offender. Severity of involvement varies widely, from mild
and transient hepatitis, to fulminant hepatic failure requiring liver
transplantation. Early identification of patients at highest risk has proved
difficult, but there are indications that certain clinical markers (such as the
presence of atypical targets and purpura at presentation) and specific high‐notoriety
drugs (such as minocycline) may confer a higher risk of more severe hepatic
involvement. Liver dysfunction is the primary cause of mortality from DRESS.
Up
to 10% patients manifest renal involvement in the course of their illness.
Again, certain drugs confer a higher risk of kidney injury, notably
allopurinol. Histologically, interstitial nephritis is seen. Cardiac
involvement, both pericarditis and myocarditis, is reported in DRESS.
Pulmonary
involvement in DRESS reveals pleural effusion, pleuritis or acute interstitial
pneumonitis.
Central
nervous system involvement in DRESS includes inflammation of the meninges and
encephalitis.
Gastrointestinal
involvement in DRESS includes ulcerative colitis and esophagitis.
Endocrine
system is usually involved in the latter phase of DRESS than the acute phase,
with the thyroid gland being most frequently involved. Both hyper‐
and hypothyroidism are recognized in the convalescent phase, both of which may
have a chronic course, and therefore regular monitoring of thyroid function for
a year after the acute event is advocated.
Other
autoimmune phenomena such as alopecia areata and SLE have also been described
in the aftermath of DRESS.
Clinical
variants
The
preferred Diagnostic criterion is that proposed by the RegiSCAR group.
|
RegiSCAR SCORING SYSTEM FOR DRESS |
|||
|
Criteria |
No |
Yes |
Unknown/unclassifiable |
|
Fever (≥38.5°C) |
-1 |
0 |
−1 |
|
Lymphadenopathy (≥2 sites; >1 cm) |
0 |
1 |
0 |
|
Circulating atypical lymphocytes |
0 |
1 |
0 |
|
Peripheral hypereosinophilia |
0 |
0 |
|
|
0.7–1.499 × 109/L - or -
10–19.9%* |
1 |
||
|
≥1.5 × 109/L - or - ≥20%* |
2 |
||
|
Skin involvement |
|||
|
Extent
of cutaneous eruption > 50% BSA |
0 |
1 |
0 |
|
Cutaneous
eruption suggestive of DRESS** |
-1 |
1 |
0 |
|
Biopsy
suggests DRESS |
-1 |
0 |
0 |
|
Internal organs involved† |
0 |
0 |
|
|
One |
1 |
||
|
Two or more |
2 |
||
|
Resolution in ≥15 days |
-1 |
0 |
−1 |
|
Laboratory results negative for at least three of the following
(and none positive): (1) ANA; (2) blood cultures (performed ≤3 days after hospitalization); (3) HAV/HBV/HCV serology; and (4) Chlamydia and Mycoplasma serology |
0 |
1 |
0 |
|
Final score: < 2, no case; 2–3, possible case; 4–5, probable
case; >5, definite case |
|||
* If
leukocytes <4.0 × 109/L
** At least
two of the following: edema, infiltration, purpura, scaling.
† Liver,
kidney, lung, muscle/heart, pancreas, or other organ and after exclusion of
other explanations.
Liver:
transaminases >2 × upper limit of normal (ULN) on two successive dates or
bilirubin × 2 ULN on 2 successive days or aspartate aminotransferase (AST), γ‐glutamyltransferase
(GGT) and alkaline phosphatase >2 × ULN on one occasion. Renal: creatinine
1.5 × patient's baseline. Cardiac: echocardiographic evidence of pericarditis.
|
|
|
|
|
|
Japanese
Research Committee on Severe Cutaneous Adverse [Drug] Reactions (J-SCAR)
diagnostic criteria for DIHS (drug-induced hypersensitivity syndrome)/DRESS
(drug reaction with eosinophilia and systemic symptoms).
Typical
DIHS is defined as the presence of all 7 criteria, while atypical DIHS is
defined as the presence of only the first 5 criteria.
|
J-SCAR DIAGNOSTIC CRITERIA FOR DIHS/DRESS |
|
1.
Maculopapular rash developing >3 weeks
after starting therapy with a limited number of drugs 2.
Prolonged clinical symptoms after
discontinuation of the causative drug 3.
Fever (>38°C) 4.
Liver abnormalities (ALT >100 U/L)* 5.
Leukocyte abnormalities (at least one
present): (a) leukocytosis (>11 × 109/L); (b)
atypical lymphocytes (>5%); and (c) eosinophilia (>1.5 × 109/L) 6.
Lymphadenopathy 7.
HHV-6 reactivation |
* This can
be replaced by other organ involvement, such as renal involvement.
Differential
diagnosis
The
other SCAR syndromes should be considered in the differential diagnosis of
DRESS. Certain clinical features are common to different SCAR syndromes, such
as pustules, which when present should provoke consideration of AGEP as a
differential diagnosis. However, the pustules in AGEP tend to be predominantly
flexural, whereas in DRESS they are unlikely to be localized to these sites.
Epidermal loss, purpura and target lesions of the skin may be present in DRESS,
all of which may occur in EM, SJS or TEN. One of the most helpful features in
distinguishing DRESS from the other SCAR syndromes is latency of onset of the
eruption; this is classically shorter in AGEP (<5 days) and SJS/TEN (7–10
days) than in DRESS, where the latency may be 2–6 weeks after drug ingestion.
Classification
of severity
Walsh
et al. classified the eruption seen in DRESS morphologically into four categories:
urticated papular exanthem, morbilliform erythema, exfoliative erythroderma and
an EM‐like reaction. The EM‐like
group demonstrated more pronounced liver dysfunction with high mortality.
A
number of hematological markers such as eosinophilia, pancytopenia and
thrombocytopenia are poor prognostic indicators.
Complications
and co‐morbidities
The
most severe and life‐threatening complication of DRESS is
fulminant liver failure, necessitating transplant. Mortality has been estimated
at 5–10%, with hepatic failure being the predominant cause of death. A delayed‐onset
interstitial nephritis is described, following cases of DRESS where kidney
involvement has been prominent, and, analogous to this, a persistent
interstitial pneumonitis is also described where pulmonary involvement has been
present. Thyroid dysfunction may supervene in the convalescent phase of DRESS.
Myocarditis, with associated cardiac insufficiency has also been described.
Autoimmune
phenomena may arise following DRESS, including lupus erythematosus and
autoimmune thyroid disease. One author has described detection of
autoantibodies in the convalescent phase in 44% patients with DRESS syndrome.
The autoantibodies detected were antinuclear antibody (ANA), antithyroglobulin
antibody (ATGA) and antithyroperoxidase antibody (ATPOA). There is
preponderance of autoantibody positivity in the non‐corticosteroid
treated group as compared to the group who received corticosteroid, suggesting
a possible protective role for this treatment.
A
small number of patients may develop chronic exfoliative dermatitis.
Disease
course and prognosis
The
majority of patients with DRESS will recover fully, following withdrawal of the
culprit drug and management of the acute episode. A number of organ‐specific
chronic sequelae may arise following involvement of these organs in the acute
phase. In addition, a number of autoimmune phenomena such as alopecia areata
and autoimmune thyroid disease are described.
Investigations
Assessment
and longitudinal evaluation of patients with DRESS (drug reaction with
eosinophilia and systemic symptoms).
BM, bone marrow; BUN, blood urea nitrogen; CBC,
complete blood count; CK, creatine kinase; CRP, C-reactive protein; LDH, lactic
dehydrogenase; LFTs, liver function tests; PFT, pulmonary function test; PT,
prothrombin time; PTT, partial thromboplastin time; TSH, thyroid stimulating
hormone.
|
ASSESSMENT AND LONGITUDINAL EVALUATION OF PATIENTS WITH DRESS
(DRUG REACTION WITH EOSINOPHILIA AND SYSTEMIC SYMPTOMS) |
|
Basic laboratory screening during the acute phase with
recommended repetitive tests in italics^ |
|
·
CBC with differential, platelet count, peripheral
smear for atypical lymphocytes ·
BUN, creatinine, urinalysis, spot
urine for protein : creatinine ratio* ·
LFTs, creatine kinase (CK), lipase,
CRP ·
TSH, free T4 (repeat
at 3 months, 1 year, and 2 years) ·
Fasting glucose (in anticipation of
systemic corticosteroids) |
|
Additional testing |
|
·
ECG, troponin T, baseline echocardiogram ·
Quantitative PCR for HHV-6, HHV-7, EBV, CMV ·
Wright stain of urine for eosinophilia
(prior to instituting corticosteroids) ·
ANA, blood cultures (exclusion criteria in
RegiSCAR scoring system) ·
If hemophagocytic lymphohistiocytosis
suspected**, ferritin, triglycerides, LDH, BM examination |
|
Further testing based upon laboratory abnormalities or signs and
symptoms** |
|
·
Liver – PT, PTT, albumin ·
Renal – albumin, renal ultrasound (if
laboratory abnormalities) ·
Cardiac – ECG, troponin T, echocardiogram ·
Neurologic – brain MRI ·
Pulmonary – CXR, PFTs ·
Gastrointestinal – endoscopy |
^ Testing
is more frequent during the acute phase (e.g. twice weekly) with frequency also
a reflection of disease severity. Longitudinal evaluation is recommended for at
least one year.
* Allows
for immediate assessment for proteinuria.
** Including
during longitudinal evaluation.
Treatment
The
most important initial task is to identify and stop the culprit drug, but this may not result in a rapid complete recovery.
The
mainstay of active treatment is corticosteroid therapy, administered topically,
orally or intravenously. In refractory cases, or where the disease enters a
chronic phase, a steroid‐sparing agent such as cyclosporine may be
required.
First
line
In
cases of limited severity with minimal cutaneous involvement, or where
administration of systemic corticosteroid is contraindicated, the application
of highly potent topical steroids may suffice as treatment. However, the
majority of patients will require systemic corticosteroid therapy, either via
the oral route or the intravenous route, as guided by clinical state. A dose
oral prednisolone of 1 mg/kg/day is recommended as initial treatment. Because relapse can occur when the dosage is reduced, a
slow taper of corticosteroids over a period of several weeks to months (on an
average 1 to 3 months) is often required. Where intravenous therapy is required, or where
institution of oral therapy has failed to produce a satisfactory clinical
improvement, methylprednisolone is indicated using a dose of 1 g/day for 3
days.
Second
line
Cases
of DRESS refractory to steroid treatment may require cyclosporine, and is
useful in patients where a protracted course of illness (e.g. with persistent
liver dysfunction or a chronic exfoliative dermatitis) supervenes.
Prevention
The individual must
be aware of his or her specific drug hypersensitivity and that other drugs of
the same class can cross-react. These drugs must never be readministered.
Patient should wear a medical alert bracelet.
Acute generalized
exanthematous pustulosis (AGEP)
Definition
Acute
generalized exanthematous pustulosis (AGEP) is one of the severe cutaneous
adverse reaction (SCAR) syndromes. It is an acute febrile drug eruption
characterized by numerous small primarily non-follicular, sterile pustules,
arising within large areas of edematous erythema, usually localized to the
major flexures. It is a self‐limiting phenomenon,
which usually resolves without sequelae. It has short latency, rapid onset and
resolution, and full recovery without recurrence. More than 90% of cases of
AGEP are drug-induced. Occasionally, it may be due to other causes, e.g. an
enteroviral infection or exposure to mercury.
Epidemiology
The
incidence of AGEP is estimated to be 1–5 per million per year.
Age
AGEP occurs more
frequently in the adult population with a mean age of onset is 56 years.
Sex
A slight female
preponderance is noted.
Pathogenesis
HLA-B5,
-DR11 and -DQ3 are found more frequently in patients with AGEP, and mutations in IL36RN may be a
risk factor as well. A prior sensitization (including a contact
sensitization) would explain the short interval (<4 days) between drug
administration and the onset of the eruption, as this suggests an immunologic
recall phenomenon. The percentage of positive patch
tests to an incriminated drug is relatively high (50–60%). Blood neutrophilia
and the accumulation of neutrophils within the lesions suggest the release of
neutrophil-activating cytokines by drug-specific T lymphocytes (e.g. IL-3,
IL-8, IL-17, G-CSF), but the precise underlying mechanisms of AGEP are still
unknown.
Antibiotics are the
primary drugs implicated in AGEP.
Commonest drugs
causing AGEP:
|
AGEP – MOST COMMONLY ASSOCIATED MEDICATIONS |
|
|
Acetaminophen Antibiotics ·
Penicillins, aminopenicillins ·
Cephalosporins ·
Clindamycin ·
Pristinamycin ·
Sulfonamides ·
Metronidazole ·
Carbapenems ·
Quinolones ·
Macrolides |
·
Calcium channel blockers, especially diltiazem ·
Carbamazepine ·
Cetirizine ·
Herbal medicines ·
Antimalarials, especially
hydroxychloroquine ·
NSAIDs, including oxicam derivatives and
COX-2 inhibitors ·
Proton pump inhibitors ·
Terbinafine |
Common culprits are
in italics.
Clinical
features
AGEP is
characterized by a high fever and pustular rash. After
drug administration, it may take 1 to 3 weeks before skin lesions appear.
However; in previously sensitized patients, the skin symptoms may occur within
2 to 3 days. This short latency is typical of AGEP. A prodrome of burning or
itching in the skin may be described, and the patient may be asthenic.
Small (<5 mm), primarily non-follicular, yellow sterile pustules appearing suddenly on a diffuse, edematous erythematous skin,
usually starts either on the face or in the major flexural creases such as the neck,
axillae and inframammary and inguinal folds, and then rapidly disseminated over a few hours to involve the trunk and
upper limbs. Typically, there are > 100 pustules which may be irregularly
dispersed or grouped. The palms and soles are spared. Edema of the face and hands,
purpura, vesicles, bullae, erythema multiforme-like lesions, and/or mucous
membrane involvement is observed in ~50% of patients. Pustules
resolve spontaneously in < 15 days and generalized post pustular superficial desquamation occurs approximately 2
weeks later.
A set of diagnostic
criteria was proposed by Roujeau et al. in 1991 as follows:
1.
Appearance
of hundreds of sterile non‐follicular pustules
at flexural sites.
2.
Histopathological
changes of spongiosis and epidermal pustule formation.
3.
Fever
>38°C.
4.
Blood
neutrophil count >7 × 109/L.
5.
Acute
evolution.
Pathology
Histologically,
both spongiform sub corneal and/or intra epidermal pustules, a marked edema of
the papillary dermis and a perivascular mixed infiltrate with neutrophils and
some eosinophils are usually present. CSVV and focal necrotic
keratinocytes are unusual findings.
Differential diagnosis
AGEP must be
differentiated from acute pustular psoriasis of the von Zumbusch type. The
pustules are clinically indistinguishable, but additional skin lesions such as
petechiae, purpura, atypical target-like lesions and vesicles are more
frequently observed in AGEP. Histologically, massive edema in the superficial
dermis, exocytosis of eosinophils, vasculitis, and necrosis of keratinocytes
are all suggestive of AGEP, whereas acanthosis is more characteristic of
pustular psoriasis. The two major differences are the acuteness (rapid appearance)
of the disease and the history of recent drug exposure in AGEP. However, AGEP
may occur more frequently in patients with a history of psoriasis, thus making
the distinction even more difficult.
A
relevant drug history, the lack of a personal or family history of psoriasis
and the absence of other stigmata of psoriasis on clinical examination may be
helpful in directing the clinician to diagnosis of AGEP rather than psoriasis.
Features which distinguish AGEP from pustular
psoriasis
|
|
AGEP |
Psoriasis |
|
History of psoriasis |
Possible |
Common |
|
Distribution pattern |
Predominance in the folds |
More generalized |
|
Duration of pustules |
Shorter |
Longer |
|
Duration of fever |
Shorter |
Longer |
|
History of drug reaction |
Usual |
Uncommon |
|
Recent drug administration |
Very frequent |
Less frequent |
|
Arthritis |
Rare |
∼30% |
|
Histology |
Spongiform subcorneal and/or
intraepidermal pustules, edema of papillary dermis, vasculitis, exocytosis of
eosinophils, single‐cell necrosis of keratinocytes |
Subcorneal and/or intraepidermal
pustules, papillomatosis, acanthosis |
Subcorneal
pustular dermatosis (Sneddon–Wilkinson disease) may be distinguished by its
less acute course, and the presence of flaccid pustules which demonstrate a
hypopyon.
Common exanthematous drug eruptions may have a few pustules, but
they are usually follicular. In severe forms of AGEP, the confluence of the
pustules and subsequent superficial epidermal detachment may lead to confusion
with TEN. However, the presence of subcorneal pustules in biopsy specimens
allows one to distinguish between the two entities.
In addition to facial edema, pustules may also be observed in
DRESS, however
pustules will generally be less numerous in DRESS than in AGEP, and the prolonged evolution, atypical lymphocytosis,
marked hype eosinophilia, and common visceral involvement usually permit
differentiation.
Investigations
In most cases, a
careful drug history is adequate to elucidate the culprit drug, which must be
excluded as a matter of priority in the care of these patients. A skin biopsy
should be taken early in the disease presentation as it will assist in the
distinction from pustular psoriasis. Baseline hematological investigations
reveal a marked leukocytosis with an elevated neutrophil count and mild to
moderate eosinophilia. Biochemical investigations should be performed to rule
out renal and liver dysfunction, as well as hypocalcemia. Measurement of acute
phase reactants such as C‐reactive protein (CRP) may help to
distinguish infection from the systemic involvement in AGEP. A septic screen
may be instituted if suspicion of infection is high.
Management
Management
of AGEP generally involves corticosteroid therapy, the route of administration
being determined by the severity of the presentation. In cases where the
patient appears systemically well, with limited areas of involvement, potent
topical corticosteroid may suffice. In cases of more extensive involvement, or
where systemic features such as fever, hemodynamic compromise or systemic upset
are seen, oral corticosteroids may be required. Emollient therapy should be
prescribed, and continued throughout the phase of post pustular desquamation,
until full skin integrity is restored.
In cases where
systemic involvement such as renal impairment or liver function disturbance is
noted, appropriate supportive care such as intravenous fluids and careful
hemodynamic monitoring should be employed. If the patient is febrile, care
should be taken to exclude an infective source, and if suspicion of this
remains, then empiric antibiotic therapy should be considered.
STEVENS -JOHNSON SYNDROME AND TOXIC EPIDERMAL NECROLYSIS
Salient features
·
Prodrome
of upper respiratory tract symptoms, fever, and painful skin
·
Stevens–Johnson
syndrome (SJS) and toxic epidermal necrolysis (TEN) are two rare, potentially
fatal, adverse cutaneous drug reactions of differing severity, characterized by
mucocutaneous tenderness and erythema as well as extensive exfoliation
·
SJS
is characterized by <10% body surface area of epidermal detachment, SJS/TEN
overlap by 10–30%, and TEN by >30%
·
The
medications most frequently incriminated are allopurinol, nonsteroidal
anti-inflammatory drugs, antibiotics, and aromatic anticonvulsants; TEN and SJS
usually occur 7–21 days after initiation of the responsible drug
·
The
average mortality rate is 1–5% for SJS and 25–35% for TEN; the mortality rates
are often higher in the elderly and those with a very large surface area of
epidermal detachment
·
Exfoliation
is due to extensive death of keratinocytes via apoptosis; the latter is
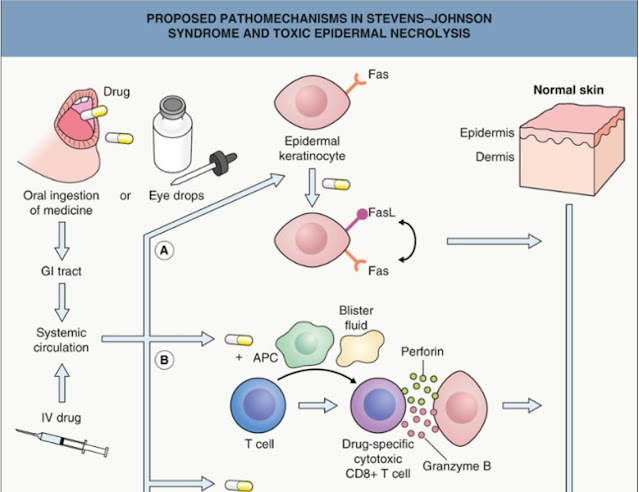
mediated via the cytotoxic secretory protein granulysin and interaction of the
death receptor–ligand pair Fas–FasL
·
Optimal
medical management of SJS and TEN requires early diagnosis, immediate
discontinuation of the causative drug(s), and rapid initiation of supportive
care
·
Additional
therapies include IVIg, cyclosporine, pulse corticosteroids, and targeted
immunomodulators, but to date none have proven efficacy based upon prospective
controlled trials
Introduction
SJS and TEN are, acute life-threatening muco-cutaneous reaction that are
almost always drug related, characterized by extensive necrosis and detachment
of the epidermis and mucous membrane, accompanied by systemic disturbance. Because of the similarities in etiology, risk factors,
histopathology, pathogenesis and clinical findings, these two conditions are
now considered as severe variants of an identical process that differs only in
the final extent of body surface involved. Therefore, it is better to use the
designation epidermal necrolysis (EN) for both. TEN is a maximal variant
of SJS differing only in the extent of body surface involvement. The disease runs an
unpredictable course. An initially benign-appearing dermatosis can progress
rapidly, and once overt skin detachment has occurred, it is difficult to
determine when it will end.
As prognosis is
correlated with the speed at which the culprit drug is identified and
withdrawn, it is crucial to establish the correct clinical diagnosis rapidly,
so the causal drug(s) can be discontinued and appropriate medical treatment
begun as soon as possible.
High-quality
supportive treatment, ideally in intensive care units with modern equipment and
trained nursing staff, is the current standard of care and can improve outcome.
No specific therapy for SJS and TEN has yet shown efficacy in prospective,
controlled clinical studies, in part because the low incidence of SJS and TEN
plus their life-threatening potential make randomized clinical trials difficult
to perform.
EPIDEMIOLOGY
Incidence
The incidence of
SJS/TEN is approximately one to two cases per million per year. But in India incidence could be much higher because the main etiologic
agents of TEN, i.e. drugs such as antibiotics, anticonvulsants, and
nonsteroidal anti-inflammatory drugs (NSAIDs) are easily available without any
prescription.
Age of Onset
EN can occur at any
age, with the risk increasing with age after the fourth decade, and more
frequently affects women.
Risk Factors
Certain patient
groups have an increased risk of developing SJS/TEN, including those who have
slow acetylator genotypes, lupus erythematosus (systemic LE or
subacute cutaneous LE), immunocompromised (e.g. HIV infection, lymphoma),
are undergoing radiotherapy while concomitantly receiving anticonvulsants, or
have specific human leukocyte antigen (HLA) alleles. Examples of the latter are
HLA-B*1502 in Asians and East Indians (but not Europeans) who are exposed to
carbamazepine and HLA-B*5801 in Han Chinese exposed to allopurinol.
Consequently, the FDA recently recommended genotyping of all Asians for the
allele HLA-B*1502 prior to the administration of carbamazepine. In individuals
with AIDS, the risk of developing TEN is 1000-fold higher than in the general
population.
|
STEVENS–JOHNSON
SYNDROME (SJS) AND TOXIC EPIDERMAL NECROLYSIS (TEN): EPIDEMIOLOGY AND RISK
FACTORS |
|
|
Annual
incidence |
1.2–6
per million (SJS) |
|
0.4–1.2
per million (TEN) |
|
|
Ratio
women : men |
1.5 : 1 |
|
Risk
factors |
· Slow acetylator
genotypes |
|
· Immunosuppression
(e.g. HIV infection, lymphoma) |
|
|
· Concomitant
administration of radiotherapy and anticonvulsants (most commonly, those with
brain tumors) |
|
|
· HLA-B*1502: Asians
(in particular Han Chinese, Thai, Malaysian populations) and East Indians
exposed to carbamazepine |
|
|
· HLA-B*5801: Han
Chinese exposed to allopurinol |
|
|
· HLA-A*3101:
Europeans exposed to carbamazepine |
|
ETIOLOGY
The reaction is independent of the dose of the drug and is idiosyncratic.
More than 100 different drugs have been implicated to date, but about a dozen
‘high risk’ medications account for one half of EN cases. These high risk drugs
are antibacterial sulphonamides, aromatic anticonvulsants, allopurinol,
NSAIDs (primarily oxicam derivatives and diclofenac), lamotrigine
and nevirapine.
The risk seems confined to the first 8 weeks of treatment. Furthermore drugs with long half-lives are more likely to cause drug
reactions and fatal outcome than those with short half-lives, even they are
chemically related.
Medications and the Risk of Toxic Epidermal Necrolysis
High Risk
·
Antibacterial
sulphonamides (Co-trimoxazole, Sulfadiazine, Sulfapyridine, sulfadoxine,
Sulfasalazine)
·
Anticonvulasants ( Carbamazepine,
Phenobarbital, Phenytoin, Lamotrigine)
·
NSAIDs (especially
pyrazolon derivatives, e.g. phenylbutazone and oxicam derivatives such as
pyroxicam)
·
Antitubercular-
Thiacetazone
·
Allopurinol
Lower Risk
·
Acetic acid NSAIDs
(e.g., diclofenac)
·
Non- sulphonamide
antibiotics (Cephalosporins, Aminopenicillins, Fluroquinolones, Tetracyclins
and Macrolides)
Doubtful Risk
·
Paracetamol
(acetaminophen)
·
Corticosteroids
·
Other NSAIDs (except
aspirin)
No Evidence of Risk
Aspirin, Sulfonylurea antidiabetic, Thiazide diuretics, Furosemide, Aldactone, calcium channel blockers, B- blockers, ACE inhibitors, Angiotensin 2 receptor antagonists, statins, hormones and vitamins.
PATHOGENESIS
The reason why some individuals develop such marked immune reactions
against medications has until very recently been obscure. A widely accepted
hypothesis has been that patients suffering from severe drug reactions are
exposed to increased amounts of reactive (oxidative) metabolites because of a
lowered ability to detoxify reactive metabolites, due to defect of the
detoxifying system in the liver and skin, either on a genetic basis, or on a
some enzyme deficiency. Such metabolite acts as a heptan and binds with the
surface of the keratinocytes and makes them antigenic. As a result, drug
specific cytotoxic CD8+ lymphocytes are formed and are responsible for massive
keratinocyte cell death via apoptosis leading to epidermal necrolysis.
Cytotoxic
T cells expressing the skin-homing receptor, cutaneous lymphocyte-associated
antigen (CLA), are seen early in the development of cutaneous lesions. These
are likely to be drug-specific cytotoxic T cells. Important cytokines such as
interleukin (IL)-6, TNF-α, interferon-γ, IL-18, and Fas ligand (FasL) are also present in the
lesional epidermis and/or blister fluid of patients with TEN, and their actions
could explain some of the constitutional symptoms of TEN as well as the
frequently observed discrepancy between the extent of epidermal damage and the
paucity of the inflammatory infiltrate. Lastly, the typical interval between
the onset of drug therapy and SJS/TEN is between 1 and 3 weeks, suggesting a
period of sensitization and providing further support for the role of the
immune system in disease pathogenesis. This period (“memory”) is considerably
shortened in patients who are unfortunately re-exposed to a drug that
previously resulted in SJS or TEN.
The
tissue damage described by pathologists as epidermal necrolysis is a reflection
of massive keratinocyte cell death via apoptosis. The apoptosis of individual
keratinocytes is a hallmark of the early stages of SJS and TEN, and it is the
first clear morphologic sign of specific tissue damage. The more classic
histologic image of extensive epidermal “necrolysis” is, in fact, the aftermath
of keratinocyte apoptosis. Indeed, the apoptotic state of cells is transient in
nature, and it is followed by necrosis if the apoptotic cells are not rapidly
phagocytosed. In SJS and TEN, within hours keratinocyte apoptosis becomes very
abundant in lesional skin, thus rapidly overwhelming the phagocytic capacity of
the affected skin. Over a period of hours to days, these apoptotic
keratinocytes become necrotic and lose their cohesion to adjacent keratinocytes
as well as the basement membrane. The entire epidermis then loses viability,
creating the familiar histologic image of full-thickness epidermal necrolysis.
SJS/TEN is primarily
a drug‐induced phenomenon, with a culprit drug being
demonstrated in approximately 85% of cases. The disease is characterized by
widespread epithelial keratinocyte necrosis of both skin and mucous membranes,
a process initiated by drug‐induced cytotoxic T
lymphocytes (CTLs). MHC class I‐restricted drug
presentation leads to clonal expansion of CD8+ CTLs which infiltrate the skin,
while soluble factors induce keratinocyte apoptosis. Pro‐apoptotic
molecules, including tumour necrosis factor‐α, interferon‐γ,
and inducible nitric oxide synthase, may link drug‐induced
immune responses to keratinocyte damage. Soluble Fas ligand, perforin and
granzyme have all been implicated in triggering keratinocyte death; however
current evidence favors granulysin as the key mediator of apoptosis in SJS/TEN.
In at least 15% of
SJS/TEN cases a culprit drug is not identified. In SJS, some cases, especially
in children, appear to be triggered by infections, most notably by Mycoplasma
pneumonia.
In
summary, the current model for the pathogenesis of SJS/TEN is as follows: upon
exposure to certain types of drugs, an individual with particular predisposing
factors mounts a specific immune reaction to the drug or one of its
metabolites. As a result of an interplay of cell types and cytokines that
remains to be totally defined, there is a strong expression of the cytolytic
molecule FasL on keratinocytes as well as secretion of granulysin from CTLs, NK
cells and NKT cells and annexin A1 from monocytes. This leads to FasL- and
granulysin-mediated apoptosis and/or annexin-dependent necroptosis of
keratinocytes and subsequent epidermal necrosis and detachment.
Proposed pathomechanisms to explain apoptosis of
epidermal keratinocytes in Stevens–Johnson syndrome (SJS) and toxic epidermal
necrolysis (TEN)
A number of
medications can trigger widespread apoptosis of epidermal keratinocytes in
individuals with SJS or TEN, leading to skin blistering and denudation. Several
theories have been proposed for this condition: (A) the medication might induce
upregulation of FasL by keratinocytes constitutively expressing Fas, leading to
a death receptor-mediated apoptotic pathway; (B) the drug might interact with
MHC class I-expressing cells and then drug-specific CD8+ cytotoxic T
cells accumulate within epidermal blisters, releasing perforin and granzyme
B that kill keratinocytes; (C) drug activated monocytes could secret annexin
A1, which induces necroptosis in keratinocytes via binding to formal peptide
receptor1(FPR1); and (D) the drug may also trigger the activation of CD8+
T cells, NK cells and NKT cells to secret granylosin, with keratinocyte death
not requiring cell contact. IVIg contains antibodies against Fas that can block
the binding of Fas L to Fas.
CLINICAL MANIFESTATION
For most drugs time from first drug exposure to onset of symptoms: 1-3
weeks, but usually develops within the first week of antibiotic therapy and up
to 2 months after starting an anticonvulsant. Prodromes with flu-like symptoms
such as fever, headache, rhinitis, cough or malaise may precede the
mucocutaneous lesions by 1–3 days. Pain on swallowing, burning or stinging of
the eyes progressively develops, heralding mucous membrane involvement. About
one third of cases begin with flu-like symptoms, one third with symptoms of
mucous membrane involvement and one third with an exanthema. Whatever the
initial symptoms are their rapid progression, the addition of new signs, severe
skin pain and tenderness, which is often out of proportion to physical findings
in early disease and constitutional symptoms, should alert one of a severe
disease.
Skin Lesions
The
earliest skin lesions appear as erythematous, dusky
red or purpuric macules of irregular size and
shape, and they have a tendency to coalesce. Lesions increase in size
and number over 5–7 days, tending to coalesce. Large areas of confluent
erythema may develop; either de novo, or from confluence of individual discrete
necrotic macules. In the absence of spontaneous
epidermal detachment, a Nikolsky sign should be sought by exerting tangential mechanical
pressure with a finger on several erythematous zones. This sign is considered
positive if dermal–epidermal cleavage is induced. In some patients, the macular
lesions observed at the onset can have a dusky center and a slightly paler
outer ring, giving them a target-like appearance
(flat atypical targets). However, such lesions lack the three concentric rings
characteristic of typical target lesions and are not papular as in atypical
target lesions of EM.
As the epidermal involvement progresses towards full
thickness necrosis, the dusky red macular lesions take on a characteristic grey
hue. This
process can be very rapid (hours), or take several days. The necrotic epidermis then detaches from the underlying dermis, and fluid
fills the space between epidermis and the dermis, giving rise to blisters.
These blisters are flaccid, break easily and extend sideways by slight pressure
of the thumb as more necrotic epidermis is displaced laterally (Asboe-Hansen
sign). Tense
blisters are usually seen only on the palmoplantar surfaces, where the
epidermis is thicker and, therefore, more resistant to mild trauma. The full-thickness epidermis is easily detached at pressure points, such as
the back, shoulders or buttocks, or by frictional trauma like a boiled potato leaving areas of
exposed dermis. The combination of both detached and
detachable epidermis (positive Nikolsky sign) will produce large areas of dark red bleeding dermis, resembling second-degree thermal
burn and
can become secondarily infected. In other areas the
pale necrotic epidermis remains in situ, with a wrinkled appearance that
resembles wet cigarette paper.
Distribution
Skin lesions tend to appear first on the upper trunk, spreading to the
neck, face and proximal upper limbs in a symmetrically distribution. Subsequently, lesions
spread to involve the rest of the trunk and limbs and becoming confluent; involvement of the palms and soles with flat atypical target
lesions is often prominent. Dusky erythema of periungual skin is commonly seen. Palms and soles may be less severely involved but the hairy portion of the
scalp is never affected. SJS:
widely distributed with prominent involvement of trunk and face. TEN:
generalized, universal.
Mucous Membranes
Involvement
of the mucous membranes of the eyes, mouth, nose and genitalia is usually an
early feature and leads to an erosive and hemorrhagic mucositis. Almost 90% of patients develop
erosive mucous membrane lesions, and can precede or
follow the skin eruption (nearly always on at least two sites). The most
frequently affected mucosal membrane is the oropharynx, followed by the eyes
and genitalia.
Oral
Oral
involvement is characterized by painful mucosal erythema with subsequent
blistering and erosions. Similar changes to the vermillion of the lips progress
to the retention of thick adherent hemorrhagic crusts. The tongue and palate
are frequently affected. In severe cases mucosal involvement may extend to the
oropharynx, larynx, respiratory tract and esophagus. Drinking and eating are
usually severely compromised by oral involvement in acute EN.
Eyes
Most patients develop intense conjunctivitis manifested by pain,
photophobia, lacrimation, redness and discharge with a tendency to form
adhesions. In less than 24 hours, the conjunctiva and cornea may become
adherent. Severe forms may lead to epithelial defect with corneal ulceration,
anterior uveitis, and purulent conjunctivitis. There may be shedding of the eyelashes.
Urogenital tract
Involvement
of the urogenital tract in EN is characterized by mucosal erythema, blistering
and erosions. During the acute phase, urogenital pain is prominent and urinary
dysfunction (dysuria or retention) is common. Genital erosions are
frequently overlooked in women, and may lead to synechiae.
Clinical
variants
SJS
must be differentiated from erythema multiforme major (EMM). In both EMM and
SJS, there is mucous membrane involvement and cutaneous blistering with
epidermal detachment of less than 10% body surface area (BSA). However, in EMM
the lesions consist of typical targets or raised atypical targets,
predominantly localized on the limbs and acral sites; in SJS, the lesions are
atypical flat targets with predilection for the torso. Distinguishing EMM from
SJS has causality implications: EMM is mostly related to herpes simplex virus
reactivation and rarely to drugs; SJS is usually triggered by a drug, rarely by
an infection.
Mycoplasma‐induced
SJS is reported, and in some cases (mostly children) may be characterized by a
predominance of mucous membrane involvement with little or no cutaneous
lesions. This clinically atypical form of SJS has been termed Mycoplasma
pneumoniae‐associated mucositis (MPAM).
Classification
of severity
Patients
are categorized according to type of cutaneous lesion and extent of maximal
epidermal detachment. Measurements of epidermal
detachment should include both detached and
detachable epidermis (positive Nikolsky sign) but not purely erythematous areas
(negative Nikolsky sign). The extent of skin detachment allows classification
of the patient into one of four groups:
· SJS is defined as:
epidermal detachment less than 10% BSA, plus widespread purpuric macules or
flat atypical targets.
· Overlap SJS‐TEN:
detachment of 10–30% BSA, plus widespread purpuric macules or flat atypical
targets.
· TEN with spots:
detachment greater than 30% BSA, plus widespread purpuric macules or flat
atypical targets.
· TEN without spots:
detachment greater than 30% BSA, with loss of large epidermal sheets without
purpuric macules or target lesions.
EXTRACUTANEOUS FINDINGS
·
Fever.
·
Usually mentally alert
but distress due to severe pain.
· The epithelium of the respiratory tract (tracheobronchial tree) is involved
with sloughing of epithelium caused by epithelial necrosis and erosions and
clinically manifested by dyspnea, elevated respiratory rate, bronchial hyper
secretion and cough. In most cases chest x-ray is normal on admission but can
rapidly reveal diffuse interstitial pneumonitis that can progress to acute
respiratory distress syndrome (ARDS) and is associated with poor prognosis. In the case of
respiratory abnormalities, fiber optic bronchoscopy may be useful to
distinguish a specific epithelial detachment in the bronchi from an infectious
pneumonitis, which has a much better prognosis.
· Gastrointestinal tract involvement: epithelial necrosis of the esophagus,
small bowel or colon manifesting as esophagitis, profuse diarrhea with
malabsorption, melena and even colonic perforation.
·
Renal involvement:
Proximal tubular damage results from necrosis of tubule cells by the same process
that destroys epidermal cells with proteinuria, micro
albuminuria, hematuria and even azotemia. Glomerulonephritis is rare.
LABORATORY EXAMINATIONS
Laboratory examinations are essential, not for the diagnosis of EN, but to
evaluate the severity of the disease and for daily management of the patient as for all
life-threatening conditions in ICU. The following
investigations should be done for all patients:
·
Evaluation
of respiratory rate and blood oxygenation are among the first steps to take in
the emergency room and any alteration (respiratory rate
> 20/min and pO2 < 80 mm Hg) is a sign of hypoxia due to pneumonia and
should be checked through measurement of arterial blood gas levels.
·
CBC (normocytic and
normochromic anemia and thrombocytopenia are common). Neutropenia is often
considered to be unfavorable prognostic factor. In the acute phase, there is a
transient decrease of peripheral CD4+ T lymphocytes, associated with
decreased T-cell function, which returns to normal in 7-10 days.
· Serum electrolytes Serum bicarbonate levels below 20 mg/L indicate a poor
prognosis. They usually result from respiratory alkalosis related to the
specific involvement of the bronchi and more rarely from metabolic acidosis.
·
Hypophosphatemia is
nearly constant.
·
Urinalysis (hematuria
and proteinuria indicate renal involvement).
·
Blood sugar (elevated
blood sugar and glycosuria). Release of stress hormones leads to a
hypercatobolic state and is responsible for inhibition of insulin secretion or
insulin resistance in peripheral tissues, which results in hyperglycemia, and
occasionally overt diabetes. A blood glucose level above 252mg/dl is one marker
of severity.
·
LFT (SGOT and SGPT) and
serum amylase (most probably of salivary origin) are slightly elevated in half
of the patients, but frank hepatitis may develop, induced by drugs or sepsis.
· RFT (blood urea nitrogen, serum creatinine levels may be raised due to
dehydration). Massive transdermal fluid loss is responsible for electrolyte
imbalance, hypoalbuminemia, and hypoproteinemia and prerenal azotemia. Raised
BUN is one marker of severity.
· Chest x-ray may show diffuse interstitial pneumonia.
·
Skin biopsy and HIV
(ELISA test) can be performed if indicated.
A
biopsy must be taken from lesional skin, just adjacent to a blister, for
routine histopathology. A second biopsy taken from peri‐blister
lesional skin should be sent unfixed for direct immunofluorescence to exclude
an immunobullous disorder.
At
presentation, swabs should be taken from lesional skin and sent for
bacteriology. Clinical photographs of the skin should be taken to show the type
of lesion and extent of involvement. The extent of erythema and the extent of
epidermal detachment should be recorded separately on a body map; for each
parameter the percentage of BSA involved should be estimated.
Blood
tests needed at presentation in Stevens–Johnson syndrome/toxic epidermal
necrolysis (SJS/TEN)
· Full blood count
· Urea and electrolytes
· Amylase
· Bicarbonate
· Glucose
· Liver function tests
· Erythrocyte
sedimentation rate
· C‐reactive
protein
· Coagulation studies
· Mycoplasma serology
· Antinuclear antibody
and extractable nuclear antigen
· Complement
·
Indirect
immunofluorescence
PROGNOSIS AND
CLINICAL COURSE
Course of
epidermal necrolysis (EN)
First symptoms of the reaction are non-specific like fever, sore throat,
and reduced state of health (prodromal symptoms). Medications given for the
prodromal symptoms are not associated with EN even if the clear sign (mucosal
involvement, macules) appears after their intake (“protopathic bias”; 1-3D).
After admission the progression of EN to maximum skin detachment continues for
up to 5 days (5D).
The epidermal detachment progresses for the first 5 to 7 days. With appropriate
supportive therapy, and intensive skin/mucous membrane‐directed
treatment, re‐epithelialization should start once the
disease stops extending. In most uncomplicated cases
re‐epithelialization will take 2- 3 weeks to heal eroded areas. This process results
from proliferation and migration of keratinocytes from “reservoir” sites, such
as healthy epidermis surrounding denuded areas and hair follicles within the
areas of detachment. Due to this conserved capacity for re-epithelialization,
skin grafting is not required in EN. Delayed healing will occur in the presence
of skin sepsis, systemic complications, or if the triggering agent (culprit
drug) has not been removed. Pressure points and
mucosa exhibit delayed healing. Mortality rate for SJS
is 5%, 10-15% for overlap and > 30% for TEN. On average, death occurs in every
third patient with EN, and it is mainly due to septicemia. Massive trans-
epidermal fluid loss associated with electrolyte imbalance, inhibition of
insulin secretion, insulin resistance, and onset of a hyper catabolic state can
also be contributive factors. All these complications of EN are best managed in
intensive care units. They can unfortunately culminate in adult respiratory
distress syndrome and multiple organ failure despite adequate supportive
therapy. Other causes of death are pulmonary embolism and
gastrointestinal bleeding.
In EN, there are
several factors that have been correlated with poor outcome, including
increasing age and extent of epidermal detachment. In addition, the number of
medications, elevation of serum urea, creatinine and glucose levels,
neutropenia, lymphopenia and thrombocytopenia has been statistically linked to
poor outcome. Late withdrawal of the causative drug is also associated with a
less favorable outcome. It has been estimated that prompt
withdrawal of the offending drug reduces the risk of death by 30% per day. The prognosis is not affected by the type or dose of the responsible
drug or the presence of human immunodeficiency virus infection.
A prognostic scoring system is called SCORTEN. It should be calculated at
admission and 3 days later.
Prognostic Factors
|
SCORTEN |
|
|
Prognostic factors |
Points |
|
Age >40 years |
1 |
|
Heart rate >120 bpm |
1 |
|
Cancer or hematologic malignancy |
1 |
|
BSA involved on day 1 >10% |
1 |
|
Serum urea level (>28mg/dL) |
1 |
|
Serum bicarbonate level (<20 mmol/L) |
1 |
|
Serum glucose level (>252mg/dL) |
1 |
|
SCORTEN |
Mortality rate (%) |
|
0–1 |
3.2 |
|
2 |
12.1 |
|
3 |
35.8 |
|
4 |
58.3 |
|
≥5 |
90 |
COMPLICATION AND SEQUELAE
Acute
complications
Acute complications are similar to those of extensive burns.
Patients develop acute skin failure, its main features are:
·
Barrier dysfunction: The
total daily fluid loss averages 3–4 L in adult patients with TEN affecting 50%
of body surface area. It induces a reduction of intravascular volume and
functional renal failure. If not corrected, hypovolemic may lead to hemodynamic
alterations and organic renal failure.
·
Temperature
dysregulation: Patients are usually febrile and shivering, even in the absence
of infection. Release of inflammatory cytokines (IL-1, TNF-a, IL-6) contributes
to high fever. Hypothermia is infrequent and usually a marker of severe
infection and irreversible septic shock.
·
Immune impairment:
selective depletion of CD4+ helper T cells and
·
Infection: The commonest life‐threatening
complication of acute SJS/TEN is septicemia. The denuded dermis in SJS/TEN acts
as a substrate for microbial colonization, initially by Staphylococcus aureus
and later by Gram‐negative rods from the digestive flora,
especially Pseudomonas aeruginosa. Systemic sepsis can quickly follow skin
infection and lead to multiorgan failure.
Pathogenesis of
altered temperature regulation in acute skin failure
Long‐term complications
Survivors
from an acute episode of SJS/TEN may develop delayed sequelae which are
associated with significant morbidity and reduced quality of life. The most
common sequelae involve the skin and mucous membranes; the most disabling
complications are ocular.
In EN patient’s sequelae tend to be more frequent and more severe than
previously thought. So prevention and adequate management of sequelae are as
important as saving the life in acute phase.
Long-term complications of acute skin failure
Skin
In
the skin, post‐inflammatory dyspigmentation persists in
darker skinned patients from months to years following resolution of the acute
dermatosis. Re‐epithelialization usually occurs without
scarring but cicatricial healing may develop in areas which were infected during
the acute phase and at sites of unrelieved pressure injury. Eruptive
melanocytic naevi occur occasionally in the recovery phase, more commonly in
children and young adults. Shedding of nails (onychomadesis) may occur a few
weeks after the acute episode due to nail matrix arrest; occasionally, there is
subsequent permanent anonychia. Involvement of the scalp in acute SJS/TEN is
extremely unusual, however telogen effluvium occurs in about 20% of patients in
the post‐acute phase. Other skin complications
include: pruritus, abnormal photosensitivity, abnormal sweating and heterotopic
ossification.
Eyes
Long‐term
ocular sequelae are the most disabling complications of SJS/TEN. Late ophthalmic complications are reported in about 40% of EN
survivors. The relationship between the initial severity of
ocular involvement and the development of late complications seems now to be
well established. Chronic
complications include corneal and conjunctival ulceration and scarring, dry
eye, distichiasis, entropion, trichiasis and ocular surface failure. In the
conjunctiva, scarring of the fornix obstructs the ductal openings of lacrimal
glands thus aggravating ocular dryness. Bulbar and forniceal cicatricial
changes lead to symblepharon or ankyloblepharon formation with limitation of
ocular mobility and interference of the tear meniscus. Scarring of the eyelid
margin leads to ectropion, entropion and misdirected eyelashes. Patients with
chronic eye involvement require lifelong management for dryness, conjunctival
inflammation and ocular discomfort; many suffer permanent visual impairment or
blindness. Such severe eye lesions occasionally develop in
patients who had no patent ocular signs during the acute phase of EN.
Oral mucosa
A range of long‐term
oral complications may occur resulting in functional impairments. Oral mucosal
scarring can cause gingival synechiae resulting in food trapping and limitation
of oral mobility. A Sjögren‐like syndrome has
been reported (ANA/Ro/La‐negative) and is believed to occur in up to
40% of survivors. Other complications include altered taste
and late alteration of teeth.
Lungs
The
most important late complication of pulmonary involvement is bronchiolitis
obliterans, in which airway epithelial injury is followed by regeneration and
scarring. It leads to severe airway obstruction and progressive dyspnea. Most
cases present 3–4 months after the acute episode and are associated with a poor
prognosis.
GIT
Long‐term
complications in the gastrointestinal tract are rare but esophageal stricture
is reported. Intestinal ulceration may occur in acute SJS/TEN and usually heals
along with skin re‐epithelialization, however in some patients
small intestinal ulcers can be persistent causing diarrhea and malabsorption.
Urogenital
Chronic urogenital
lesions in SJS/TEN are mostly adhesions: vaginal and introital adhesions may be
associated with dyspareunia. Other gynecological complications include vaginal
adenosis which is the replacement of non‐cornified vaginal
epithelium with metaplastic epithelium of endocervical differentiation. Phimosis occurs in male patients.
Because these late complications and sequel may develop insidiously, it is
strongly suggested that all patients surviving EN have a clinical follow up for
a few weeks after discharge and 1 year later, including examination by an
ophthalmologist and by other organ specialists as indicated by abnormal signs
and symptoms.
Pathology
Histopathologic
examination of lesional skin is a very useful tool for confirming the diagnosis
of SJS and TEN, as the morphologic findings are distinct from those observed in
SSSS (subcorneal blister with cleavage located in the granular layer of the
epidermis) and acute generalized exanthematous pustulosis (AGEP; a rich
neutrophilic infiltrate, superficial epidermal pustules and spongiosis, but no
full-thickness epidermal necrolysis). Frequently, immediate analysis of frozen
cryostat sections is sufficient for this purpose.
In early
lesions of SJS and TEN, apoptotic keratinocytes are observed scattered in the
basal and immediate suprabasal layers of the epidermis. This is most likely the
histologic correlate of the dusky to gray color that clinicians familiar with
SJS/TEN consider as a warning sign of impending full-blown epidermal necrolysis
and detachment. At later stages, lesional biopsy specimens show
a
subepidermal blister with overlying confluent necrosis of the entire epidermis
and a sparse perivascular infiltrate composed primarily of lymphocytes. At an
immunopathologic level, variable numbers of lymphocytes (usually CD8+) and macrophages are observed within the epidermis,
whereas lymphocytes in the papillary dermis are primarily CD4+ cells.
MANAGEMENT
EN is a life threatening disease that requires optimal management:
·
Early diagnosis, and
immediate withdrawal of suspected drug(s),
·
Supportive care and
·
Specific therapy.
Prompt identification and immediate withdrawal of the causative drug(s) is
associated with increased rate of survival in patients
with EN induced by drugs with short elimination half-lives. On the other hand,
it is preferable to continue every important and nonsuspected medication. That
will avoid reluctance on the part of the patient's physicians to prescribe them
in the future. Currently, there is no reliable test for the
identification of causative drugs, so the clinician, has to rely on previously
reported associations and determine the probability (unlikely, possible,
plausible, probable, very probable) for each drug based on their intrinsic
ability to cause EN and extrinsic factors such as the onset of a given
medication with respect to the onset of EN. In case of doubt, all non-life
sustaining drugs should be stopped, and particularly those administered within
the previous 8 weeks. In general, the medication list of patients with EN
should be reduced to a strict minimum, appropriate substitution made and drugs
with short half-lives favored.
Symptomatic Treatment
The
fundamental elements of patient management in SJS/TEN are meticulous care of
lesional skin and mucous membranes, coupled with intensive supportive care for
the systemic complications of acute skin failure. Only patients with
limited skin involvement, a SCORTEN score of 0 or 1, and a disease that is not
rapidly progressing can be treated in nonspecialized wards. Others should managed in an
appropriate care setting, usually an intensive care unit (ICU) or burns unit,
by a team of clinicians experienced in treating SJS/TEN. There is no “specific” treatment of demonstrated efficacy and supportive
measures are the most important. Supportive care consists of maintaining
hemodynamic equilibrium and preventing life-threatening complications.
Supportive care:
Aim of supportive care is to limit complications such as hypovolemia,
electrolyte imbalance, renal insufficiency and sepsis which are the major cause
of mortality.
·
Admit the patient to ICU
or burn unit and the room temperature should be maintained at (30-32 degree C)
to reduce heat loss through the skin.
·
Measure extent of
epidermal detachment by ‘Rule of Nines’ or the simple rule that one hand (palm
and fingers) corresponds to 1% of the BSA.
·
Place large bore
intravenous line by venous catheter in a region of non involved skin to ensure
adequate intravenous access and to prevent sepsis.
·
Replacement of IV fluids
and electrolytes is same as for patient with a third-degree thermal burn.
However, less fluid (about 70%) usually required as for thermal burn of similar
extent, because interstitial edema is absent. For the first 24 hours average
4-6 liters/day fluid and electrolyte is required (isotonic saline 0.7ml/kg/% of
body surface area (BSA) affected+ human albumin 1ml/kg/% BSA. Potassium
phosphate is added to I/V fluids to prevent insulin resistance). Half the calculated
fluid is administered in the first 8 hours and the other half in the next 16
hours. Thereafter, fluid requirement should be guided by the previous day
urinary output. After admission, an oral
liquid diet or a nasogastric tube (Ryle’s tube) should be considered in the
presence of severe mucosal involvement restricting oral intake until the oral
mucosa has healed (1500 calories in 1500ml over the first 24hrs, increasing by
500 calories daily to 3000-4000 calories day).
Proteins (approx 1.5 g/kg/day) are given to avoid negative nitrogen
balance as protein loss, from skin lesions and increased catabolism, may reach
up to 150–200 g/day.
·
Daily urinary output
measurement by Foley’s catheter and is maintained at more than 1500ml/day.
·
All lines should be checked
daily for signs of infection, changed at least every 3 days, and the tips of
all discarded lines and catheters sent for culture.
·
Monitor for infection. To reduce the risk of infection, aseptic and careful handing is
required. Treat empirically with systemic
antibiotics until c/s reports only for documented infections or signs of sepsis
(sudden rise or fall of temperature, deterioration of consciousness, fall in
urinary output, tachycardia, tachypnea, and increase in insulin requirement).
Empirical coverage should include one antibiotic having anti- staphylococcal
activity and one effective against gram negative bacteria.
·
Prophylactic anticoagulation is provided during
hospitalization.
· An hourly record of the pulse, respiratory rate, blood pressure, and urine
output is essential; glycosuria and body temperature should be recorded every 3
hours. An accurate daily intake-output chart should be maintained. A urine
output of 50-100 ml/hour is indicative of adequate tissue perfusion whereas
reduction in urine output may be an early indicator of hypovolemia or septicemia.
A pulse rate of =/> 120 /minute, even in the presence of precipitating
factors like septicemia and fever, indicates a negative fluid balance.
·
Sedation and pain
therapy.
·
Lung involvement may be
complicated by pulmonary edema during fluid replacement. Pulmonary care
includes normal saline aerosols, bronchial aspiration and postural drainage by
turning the patient to different sides.
·
Period culture of skin
erosions, blood and urine for bacteria and fungi.
·
Physical therapy daily
to preserve limb mobility and to prevent contractures.
·
Consult physician to
manage co morbidities, pulmonary medicine for airway involvement and
gastroenterology for alimentary involvement.
Skin care:
Skin care is important to minimize heat and fluid loss, prevent infections
and provide a moist environment to promote re-epithelisation.
·
All manipulation should
be performed sterilely. Move the patient as little as possible because every
movement is a potential cause of epidermal detachment. Adhesive tape should not
be applied directly to involved skin when possible.
·
Air-fluidized bed to
minimize shearing forces.
·
Non detached areas are
kept dry and not manipulated.
·
Detached or detachable
epidermis should be left in position as a biological dressing, should not be
debrided and that only denuded areas in contact with bed should be covered with
non-stick dressing such as Vaseline gauze until re-epithelisation has occurred.
Blisters are fragile, left undisturbed or may simply punctured.
·
Skin care should be
focus on the face, nose, ears, ano-genital region, axillary folds and
interdigital spaces. Clean serous and bloody crusts from these areas with
sterile normal saline daily and apply antibiotic ointment e.g. mupirocin.
·
Regular examination of
the eyes by an ophthalmologist to minimize the risk of conjunctival scarring
and blindness. Regular installation of antiseptic eye drops and separation of
newly formed synechiae are required.
·
Mouth should be rinsed
several times daily with antiseptic (chlorohexidine rinses) and antifungal
solution. For lip erosions apply white petrolatum ointment.
·
Avoid sulpha-containing
topical or systemic preparation.
·
Consult ENT specialist
to evaluate extent of upper respiratory tract involvement.
·
Consult Gynecologist and
urologist for genitourinary involvement.
·
If extensive denuded
areas, use biological dressings or skin equivalents.
Care
environment and care provision
Patients
with large areas of epidermal loss (greater than 10% of BSA) should be admitted
to an ICU for critical care management and specialist nursing. Since the
cutaneous defect in SJS/TEN is analogous to a large superficial burn, many
patients are transferred to a burns unit which can deliver both intensive supportive
management as well as skin‐directed therapy.
Rapid admission to a specialist unit improves survival, whilst a delay in
transfer is accompanied by increased mortality.
A
number of specialist services are needed to manage EN effectively. A
multidisciplinary team (MDT), coordinated by a specialist in acute skin failure
(dermatologist or burns surgeon), should be convened to manage the patient. As
well as dermatology and wound care expertise, the EN MDT must include
clinicians from intensive care, ophthalmology and skin care nursing. Additional
clinical input is often required from thoracic medicine, gastroenterology, gynecology,
urology, oral medicine, microbiology, dietetics, physiotherapy and pharmacy.
Within an ICU or burns care unit the patient must be barrier nursed in a side
room on a pressure‐relieving mattress with the ambient
temperature raised to 25–28 °C.
Skin
handling, topical therapy and dressings
In
EN, necrolytic epidermis readily detaches from underlying dermis and therefore
careful handling of the skin is essential. Day‐to‐day
bedside care should be delivered by specialist nurses familiar with skin
fragility disorders. Shearing forces applied to the skin, a particular problem
in patient positioning, should be limited. Other sources of skin trauma to be
avoided include the use of sphygmomanometer cuffs, adhesive ECG leads, adhesive
dressings and identification wrist tags.
Despite
careful nursing, lesional epidermis (referred to as ‘detachable epidermis’) in EN
often peels away, especially at pressure areas, to leave zones of denuded
dermis. In the conservative approach, detached epidermis can be left in situ to
act as a biological dressing for the underlying dermis. In cases where bullae
are prominent, it can be decompressed by fluid aspiration and the blister roof
retained to cover the underlying dermis.
The
intact skin should be cleansed each day by gentle irrigation with warmed
sterile water or sprayed with a weak solution of chlorhexidine (1/5000). If
mobility permits, the patient may be bathed in a weak solution of chlorhexidine
(1/5000). The whole skin, including denuded areas, should be treated with
frequent applications of a greasy emollient, such as 50% white soft paraffin
with 50% liquid paraffin (50/50 WSP/LP). Aerosolized formulations of 50/50 WSP/LP
can be used to minimize shearing forces associated with topical applications. A
topical antibiotic ointment should be used only on sloughy or crusted areas, or
at sites of positive microbiology swabs.
The
use of dressings on denuded areas in EN will reduce fluid and protein loss,
limit microbial colonization, help pain control and accelerate re‐epithelialization.
Silicone dressings are recommended for areas of exposed dermis, while an
absorbent non‐adherent dressing should be applied as a
secondary layer to collect exudate and protect lesional skin. Smear the surface
of dressings with 50/50 WSP/LP prior to contact with the patient.
In
the surgical approach, favored by burns specialists, biological dressings are
applied to denuded areas under a general anesthetic.
Local
therapy for eyes, mouth and uro‐genital tract
Eyes
The
eyes should be examined by an ophthalmologist as a part of the initial
assessment and daily thereafter during the acute phase. An ocular lubricant
must be applied 2‐hourly. Ocular hygiene, to remove
inflammatory debris and break down conjunctival adhesions, must be carried out
each day. A broad spectrum topical antibiotic should be used in the presence of
corneal fluorescein staining or frank ulceration. The use of topical
corticosteroid drops, supervised by an ophthalmologist, may reduce ocular
surface damage in the acute phase of EN. For patients in whom there is
extensive loss of ocular surface epithelia which is unresponsive to
conservative measures, then amniotic membrane transplantation (AMT) can be
considered. The proposed benefits of AMT in the acute phase include reduced
inflammation, enhanced re‐epithelialization, and reduction of scarring
and symblepharon formation.
Mouth
Regular
examination of the mouth must be undertaken. Apply WSP ointment frequently to
the lips; protect ulcerated intra‐oral surfaces with a
mucoprotectant mouthwash. Clean the mouth daily with warm saline mouthwashes.
Use an anti‐inflammatory oral rinse containing
benzydamine hydrochloride every 3 h, and an antiseptic mouthwash (e.g.
chlorhexidine digluconate) twice per day. In the absence of secondary
infection, consider using a topical corticosteroid four times per day (e.g.
Betnesol mouthwash 0.5 mg in 10 mL of water as a 3‐min
rinse‐and‐spit preparation).
Uro‐genital tract
Examine
the uro‐genital tract regularly throughout the acute
illness. Use WSP ointment as an emollient frequently. Use silicone sheet
dressings to eroded areas in the vulva and vagina. Consider applying a topical
corticosteroid cream with additional antimicrobial activity to the involved but
non‐eroded surfaces. Catheterizing all patients
will prevent urethral strictures.
Fluid
replacement and nutrition
Extensive
epidermal detachment will result in large insensible transcutaneous fluid
losses, compounded by decreased oral intake due to disease involvement of the
mouth. During the acute illness, replace fluids intravenously, using a
crystalloid fluid at 2 mL/kg body weight/% of BSA epidermal detachment, or
alternatively use urine output to guide fluid replacement. Establish peripheral
venous access by inserting cannula through non‐lesional skin. Over‐aggressive
fluid resuscitation may be associated with pulmonary, cutaneous and intestinal edema.
In
EN cases with significant areas of skin involvement, a nutritional regimen must
be initiated early to support metabolic disturbances, minimize protein losses
and promote healing. Enteral nutrition is preferable to parenteral nutrition
to reduce peptic ulceration and limit translocation of gut bacteria. Since
buccal mucositis in EN often precludes normal oral intake, naso‐gastric
feeding with a silicone tube should be instituted when necessary. During the
early, catabolic phase of EN 20–25 kcal/kg/day should be delivered, while
requirements in the recovery, anabolic phase increase to
25–30 kcal/kg/day.
Analgesia
EN
is characterized by cutaneous pain which is most severe at sites of epidermal
detachment. If the patient is in severe pain, then an opiate‐based
analgesia regimen using morphine delivered by an appropriate route should be
considered. Additional analgesia is often needed to address increased pain
associated with patient handling, repositioning, dressing changes and
physiotherapy: a bolus of 0.1 mg/kg of morphine may be given
intravenously, 10–20 min before commencement of the procedure. Bolus
ketamine analgosedation (0.5 mg/kg) may be useful for procedural
analgesia. Adjuvants, including γ‐aminobutyric acid
(GABA) analogues, may have an opiate‐sparing role. Topical
anesthesia of mucous membranes may facilitate placement of naso‐gastric
tubes and urinary catheters.
Additional
supportive medication
Patients
with EN are subject to stress‐related gastric or
duodenal ulceration and, if immobile, at risk of venous thromboembolism.
Gastric protection with a proton pump inhibitor is recommended if the patient
is not absorbing food. Prophylactic anticoagulation with low‐molecular‐weight
heparin is necessary, unless contraindicated.
Anemia
and leucopenia are common complications of the acute phase of EN. Neutropenia
will increase the risk of sepsis and therefore administration of recombinant
human granulocyte–colony‐stimulating factor (G‐CSF)
has been used to resist infectious complications. It has been suggested that G‐CSF
in EN may also be immunomodulatory and enhance re‐epithelialization.
Monitoring
for infection
Cutaneous
infection is a common complication of EN: it will impair re‐epithelialization
and may lead to systemic sepsis. Swabs for bacterial and Candida culture should
be taken from multiple sites, particularly sloughy or crusted areas, throughout
the acute phase of EN. Prophylactic systemic antimicrobial therapy may increase
skin colonization, particularly with Candida albicans; therefore antibiotics
should only be given if there are clinical signs of infection. The EN disease
process may be accompanied by a fever which complicates detection of secondary
sepsis. Patients should therefore be monitored carefully for other signs of
systemic infection such as confusion, hypotension, reduced urine output and
reduced oxygen saturation. The detection of sepsis may also be indicated by a
rise in CRP, a neutrophilia and an increase in skin pain.
Definite indications of
antibiotic use
1. High bacterial
count (single strain) from skin / catheter sample of urine
2. Sudden hypothermia
in a relatively stabilized patient
3. Confused mental
status, anxiety and excitement
4. Symptoms of
infection pertaining to a particular system, e.g. pneumonia/urinary tract
infection
Specific treatment in acute
stage
Because of the importance of immunologic and cytotoxic mechanisms, a
large number of immunosuppressive and/or anti-inflammatory therapies have been
tried to halt the progression of the disease, most notably systemic corticosteroid,
IVIG and cyclosporine. None has clearly proved its efficacy.
Systemic Corticosteroids
The use of systemic corticosteroids is still controversial. Some studies
found that such therapy could prevent the extension of the disease when
administered during the early phase, in high doses and
for short period, not more than 4-5 days, especially as
intravenous pulses, but late in the disease they are
contraindicated. Other studies concluded that steroids did not stop the progression of
the disease and were even associated with increased mortality and adverse
effects, particularly sepsis. Thus, systemic corticosteroids cannot be
recommended as the mainstay treatment of EN, but a large study has suggested a
possible benefit.
Intravenous
dexamethasone 1.5 mg/kg or IV solumedrol 500mg for three
consecutive days as pulse therapy within first 48 hours of
onset of rash in patient with a still limited skin surface involvement to prevent
widespread involvement.
Intravenous
immunoglobulin
If within 48-72 h of bulla onset, use high-dose IVIG, 1gm/kg/day infused
over 4 hours for 3 consecutive days. If 72 h have passed, but the patient is
still actively progressing with new lesions, IVIG may still be used. It blocks
keratinocyte necrosis by inhibiting
the Fas‐FasL interaction and apoptosis through anti‐Fas
activity. It should be avoided in patients with impaired renal
function.
Cyclosporine
Cyclosporine is a powerful immunosuppressive agent: activation of T helper
2 cytokines and inhibition of CD8+ cytotoxic mechanisms. It can halt the
progression of EN without worrisome side effects when administered early. It is
used in the dose of 3mg/kg/day orally for 10 days and then tapered. It can be
administered by breaking the soft gel capsules, mixing the content in apple or
orange juice and administered via a nasogastric tube.
Therapeutic
ladder
First line
· Withdraw culprit drug
· If epidermal loss is
>10% BSA transfer to a specialist unit (intensive care unit or burns unit)
· Institute supportive
care package, with particular attention to:
a.
Heated
environment
b.
Fluid
replacement
c.
Nutritional
regimen
d.
Analgesia
e.
Preventing/treating
infection
· Specialist skin care
nursing is essential for delivery of topical therapy/dressings
Second line
In
the early stages of the acute phase consider using:
· IVIG (0.5–1 g/kg
daily for 3–4 consecutive days), or
· Systemic
corticosteroid (e.g. prednisolone 0.5–1 mg/kg daily for 10 days, and
tapered; or IV methylprednisolone 500 mg on 3 consecutive days), or
· Cyclosporine (3 or
4 mg/kg/day in divided doses for 10 days, and tapered)
Treatment of Sequelae
Very promising treatments have now been developed
for the ocular sequelae of EN, including gas permeable scleral lenses and
grafting of autologous stem cells from contralateral limbus or mouth mucosa.
With the exception of ocular sequelae, the literature contains only case
reports related to treating sequelae. Photo protection and cosmetic lasers may
help resolve the pigmentation changes on the skin.
SUMMARY OF DRUG REACTION
·
Every drug can cause almost every reaction, but 80% of
drug eruptions are caused by B-lactam antibiotics, aspirin, NSAIDs,
sulphonamides, allopurinol and antiepileptics.
·
In all cases, immediate identification and
discontinuation of the offending agent is an essential first step. If patients
are on multiple medications and definite causal identification is not possible,
attempt to identify the most likely offending drug and discontinue it and all
unnecessary drugs. Use alternative, pharmacologically-distinct agents. In most
cases, the cutaneous reaction will resolve in 2-5 days without therapy. Some patients, however, may require topical
or systemic therapy to control the bothersome pruritus that may accompany the
reaction such as topical corticosteroid ointment and oral antihistamines.
·
Patients who experience a simple, morbilliform drug
eruption should avoid repeated exposure to the same drug or drug class in the
future; however, in cases where this cannot be avoided, and then one can
attempt a re-challenge of the medication with close monitoring. In some cases,
adverse drug reactions do not occur upon repeat exposure.
·
Patients with a history of severe cutaneous eruption,
especially with systemic complications, should avoid repeated exposure to the
same drug or drug class in the future; their first-degree relatives should also
be counseled to avoid the drug / drug class. Patients should wear a medical
alert card that may aid patients in avoiding potentially dangerous medications.
·
In the future, we may be able to predict who is at risk
of EN using genetic screening, as specific at-risk HLA alleles have been recognized
in certain populations.
Pitfalls
·
If the cutaneous reaction does not resolve as expected,
consider another diagnosis or the possibility that the offending agent has not
been discontinued.
·
Many drugs cross-react, i.e., reactions to one medication
typically predict cross-reaction to other medications in the same class. For
example, hypersensitivity to anticonvulsants such as phenytoin results in
cross-reactivity to phenobarbital and lamotrigine.
·
Look for liver, renal, and hematologic abnormalities,
particularly if drugs such as dilantin and allopurinol are possible offenders.
·
In patients with renal or hepatic insufficiency, drug
clearance may be impaired and the rash may persist longer than 3-5 days after
discontinuation.
·
A morbilliform rash may rarely be a prodrome of
Stevens-Johnson syndrome or toxic epidermal necrolysis. Monitor for skin
tenderness, bullous and mucosal involvement.