Atopic dermatitis
Salient features
·
AD is a common chronic
inflammatory skin condition that typically begins during
infancy or early childhood and is often associated with other atopic disorders
such as asthma, allergic rhinoconjunctivitis and food allergies.
·
It represents a complex genetic
disease with environmental influences and an underlying defect in the epidermal
barrier as well as associated immune dysregulation.
·
Characterized by intense pruritus and a chronically relapsing
course.
·
Acute inflammation and involvement of the cheeks, scalp and
extensor aspects of the extremities predominates in infants, shifting to
chronic inflammation with lichenification and a predilection for flexural sites
in children and adults
·
Associated with a predisposition to skin infections, especially
with Staphylococcus aureus and herpes simplex virus.
·
A proactive approach to management is recommended, including
avoidance of trigger factors, regular use of emollients, and anti-inflammatory
therapy to control subclinical inflammation as well as overt flares; targeted
immunomodulatory therapy is available for more severe disease.
Introduction
AE
is an itchy, chronic or chronically relapsing inflammatory skin condition that
often starts in infancy or early
childhood (usually before 2 years of age). Acute inflammation and involvement of the cheeks, scalp and
extensor aspects of the extremities predominates in infants and young children shifting
to chronic inflammation with lichenification and a predilection for flexural
sites in older children and adolescent/adults.
The term atopy denotes a familial tendency to develop
certain diseases (rhinoconjunctivitis, asthma, atopic eczema) on the basis of
hypersensitivity of skin and mucous membranes to environmental substances
associated with increased IgE formation and/or epithelial barrier dysfunction.
Atopic dermatitis usually occurs in people who have an 'atopic
tendency'. This means they may develop any or all of three closely linked
conditions; atopic dermatitis, asthma and hay
fever (allergic rhinitis). Often these conditions run within families with a
parent, child or sibling also affected and a positive family history is
particularly useful in diagnosing atopic dermatitis in
infants.
AD is a complex
genetic disease and is often accompanied by other atopic disorders such as
allergic rhinoconjunctivitis, asthma and food allergies. These conditions may
appear simultaneously or develop in succession. AD and food allergy have a
predilection for infants and young children, while asthma favours older
children and rhino-conjunctivitis predominates in adolescents. This
characteristic age-dependent sequence is referred to as the “atopic march”. Atopic
dermatitis is a risk factor for the future development of allergic airways
disease, possibly by percutaneous sensitization to protein antigen. In patients harbouring a
filaggrin mutation has associated clinical features such as ichythosis
vulgaris, keratosis pilaris and hyperlinear palms.
In 2003, a consensus
conference spearheaded by the American Academy of Dermatology suggested revised
Hanifin and Rajka criteria that are more streamlined and applicable to the full
range of patient ages.
|
DIAGNOSTIC FEATURES AND
TRIGGERS OF ATOPIC DERMATITIS (AD) |
|
Essential features:
must be present and are sufficient for diagnosis |
|
Pruritus 1.
·
Rubbing or scratching can initiate or exacerbate flares
(“the itch that rashes”) ·
Often worse in the evening and triggered by exogenous
factors (e.g. sweating, rough clothing) Typical eczematous
morphology and age-specific distribution patterns 2.
·
Face, neck, and extensor extremities in infants and
young children ·
Flexural lesions at any age ·
Sparing of groin and axillae Chronic or
relapsing course |
|
Important features: seen in most cases, supportive of diagnosis |
|
Onset during
infancy or early childhood Personal and/or
family history of atopy (IgE reactivity) Xerosis .
·
Dry skin with fine scale in areas without clinically apparent inflammation; often
leads to pruritus |
|
Associated features: suggestive of the diagnosis, but less
specific |
|
Other filaggrin
deficiency-associated conditions: keratosis pilaris, hyperlinear palms,
ichthyosis vulgaris Follicular
prominence, lichenification, prurigo lesions Ocular findings:
recurrent conjunctivitis, anterior subcapsular cataract; periorbital changes:
pleats, darkening Other regional
findings, e.g. perioral or periauricular dermatitis, pityriasis alba Atypical vascular
responses, e.g. mid facial pallor, white dermographism*, delayed blanch |
|
Triggers |
|
Climate: extremes of
temperature (winter or summer), low
humidity Irritants: wool/rough
fabrics, perspiration, detergents, solvents Infections: cutaneous
(e.g. Staphylococcus aureus, molluscum contagiosum) or
systemic (e.g. URI) Environmental allergies: e.g. to dust
mites, pollen, contact allergens Food allergies: .
·
Trigger in small minority of AD patients, e.g. 10–30%
of those with moderate to severe, refractory AD ·
Common allergens: egg > milk, peanuts/tree nuts,
(shell)fish, soy, wheat ·
Detection of allergen-specific IgE (via blood and skin
prick tests) does not necessarily
mean that allergy is triggering the patient’s AD *Stoking the skin with
blunt instrument leads to white streaks within 1 minute that reflects
excessive vasoconstriction. |
|
|
|
|
|
|
|
|
|
|
|
DIAGNOSTIC
GUIDELINES FOR ATOPIC DERMATITIS The UK refinement of Hanifin and
Rajka's diagnostic criteria of atopic dermatitis (eczema) In order
to qualify as a case of atopic dermatitis (eczema) with the UK diagnostic
criteria, the child: |
||||||||||||||||||||||||
|
Epidemiology
The
current prevalence of AD is approximately 10–30% in children and 2–10% in
adults.
In general, the prevalence of AD in
rural areas and low-income countries is significantly lower than in their urban
and high-income countries. This observation supports the hygiene hypothesis,
which postulates that decreased exposure to infectious agents in early
childhood increases susceptibility to allergic diseases.
Three subsets of AD based on age of onset have emerged from
epidemiologic studies:
|
|
· Early-onset type: defined as AD beginning in the
first 2 years of life and is the most common type of AD. The age of onset is between 2 and 6
months of life in 45% of affected
individuals, during the first year of life in 60%, and before 5 years of age
in 85%. Approximately half of children
with disease onset during the first 2 years of life develop allergen-specific
IgE antibodies by 2 years of age. About 60% of infants and young
children with AD go into remission by 12 years of age, but in others disease
activity persists into adolescence and adulthood. |
|
|
|
|
· Late-onset type: defined as AD that starts after
puberty. Approximately 30% of AD patients overall are in the
non-IgE-associated category, and among adults, the vast majority of such
patients are women. · Senile-onset
type:
an unusual subset of AD that begins after 60 years of age has been identified
more recently. |
Pathogenesis
The pathogenesis of
AD can be divided into three major categories: (1) epidermal barrier dysfunction; (2) immune dysregulation; and (3) alteration of the microbiome. Each of these can be modulated by
genetic and environmental factors.
Atopic dermatitis results
from defects in epidermal barrier function, immune dysregulation, and
environmental influences.
SC, stratum corneum; KLK, kallikrein; TEWL,
transepidermal water loss; TSLP, thymic stromal lymphopoietin.
Major mechanisms of AD include abnormalities
in the terminal differentiation of keratinocytes that lead to a defective
stratum corneum. A defective barrier in AD allows the penetration of allergens
and microbes, leading to IgE sensitization and type 2 cytokine productions that
drive allergic inflammation. At least two AD subtypes can be distinguished: the
first type is characterized by a strong type 2 mediated responses with high
levels of serum immunoglobulin E (IgE) and the second is a non-allergic type
characterized with normal IgE levels.
T cells and dendritic cells in atopic dermatitis
T cells are moderately increased in the dermis
of non-lesional skin. Skin homing CLA þ T cells display a Th2 profile in early
lesions and in the circulation. However, in the course of the disease,
increasing populations of Th1 cells and IFN-g and sometimes Th17 and Th22 cells
are detected in chronic lesions of AD. IL-33, IL-25 and TSLP released from
keratinocytes directly or indirectly support the type 2 response.
Interleukin-31 (IL-31), preferentially produced from Th2 cells, binds to the
IL-31 receptor (IL-31R) expressed on sensory nerves. IL-31/IL31R signaling has
recently been shown to play a critical role in the development of pruritus in
AD. Three populations of antigen-presenting cells including Langerhans cells
(LCs), monocyte-derived LC-like cells (MDLC), and inflammatory dendritic
epidermal cells (IDECs) exist in the skin. IDECs and LC-like cells have been
found to be present in both steady states and inflammatory states, and they are
present in lesional AD. LC and IDEC in AD skin do not respond to Toll-like
receptor (TLR) 2 activation and may contribute to the inability to clear S.
aureus infection.
Elevated IgE and the inflammatory response
The role of immunoglobulin E
(IgE) in AD is unknown. According
to the current consensus nomenclature by the World Allergy Organization (WAO),
the term “atopy” is tightly linked to the presence of allergen-specific IgE
antibodies in the serum against inhalants or food allergens in 70% of AD
patients, as documented by positive fluorescence enzyme immunoassays
(previously radioallergosorbent [RAST] tests) or skin prick tests. Thus, an IgE-associated
or allergic form of atopic dermatitis is also known as extrinsic AD).
The remaining 30% of AD patients who has
normal total serum IgE levels (no evidence of IgE-sensitization against
inhalants or food allergens) is categorized as having a non-IgE-associated
or non-allergic form of dermatitis (also known as intrinsic AD). The levels of IgE do not necessarily correlate with the
activity of the disease; therefore elevated serum IgE levels can only be
considered supporting evidence for the disease. Total IgE level is
significantly higher in children with coexistent atopic respiratory disease in
all age groups. Most persons with AD have a personal or family history of
allergic rhinitis or asthma and increased levels of serum IgE antibodies
against airborne or ingested protein antigens. AD usually diminishes during the
spring hay fever season, when aeroallergens are at maximum concentrations.
Intrinsic vs Extrinsic Atopic Dermatitis
Patients with intrinsic AD do not have elevated IgE levels
and filaggrin (FLG) mutation, often begins in adulthood and the immune system
exhibits Th 22 and Th 17 activation. Patients with extrinsic AD have elevated
IgE levels and filaggrin (FLG) mutation, early onset AD and Th 2 dominant the
immune response.
|
|
Intrinsic (non-allergic) |
Extrinsic (allergic) |
|
Physical exam |
comparable
|
comparable
|
|
Frequency of AD |
~25%
|
~75%
|
|
Family history |
Yes
|
Yes
|
|
Age of onset |
Late
|
Early
(childhood) |
|
Association with other atopic disease (asthma, hay fever) |
Rare
|
Common
|
|
Serum IgE level |
Normal
|
High
|
|
Skin prick tests or RAST to |
Negative
|
Positive
|
|
Foods & aeroallergens |
|
|
|
Cytokine profile |
|
|
Blood eosinophilia
Eosinophils may be major effector
cells in AD. Blood eosinophil counts roughly correlate with disease severity,
although many patients with severe disease show normal peripheral blood
eosinophil counts. Patients with normal eosinophil counts mainly are those with
atopic dermatitis alone; patients with severe atopic dermatitis and concomitant
respiratory allergies commonly have increased concentrations of peripheral
blood eosinophils. There is no accumulation of tissue eosinophils; however,
degranulation of eosinophils in the dermis releases major basic protein that
may induce histamine release from basophils and mast cells and stimulate
itching, irritation, and lichenification.
Clinical features
Skin Symptoms Pruritus is the sine qua non of atopic dermatitis— “eczema is
the itch that rashes.” The constant scratching leads to a vicious cycle of itch
→ scratch → rash
→
itch → scratch.
Pruritus
Intense pruritus is a hallmark of AD. The itch may be
intermittent throughout the day but often worse in the early evening and night
and may be exacerbated by warmth, bathing, emotional upset, exposure to irritants and allergens, changes in humidity and
excessive sweating. Pruritus may also
flares after
taking off clothing. Wool is an
important trigger; wool clothing or blankets directly in contact with skin
(also wool clothing of parents, fur of pets, carpets). The consequences of pruritus are rubbing and scratching,
prurigo papules, lichenification and eczematous skin lesions, explaining why AD
is known as the “itch that rashes”. Excoriations (linear or punctate) are
frequently present, providing evidence of scratching. Instead of scratching causing pain, in the atopic patient the “pain”
induced by scratching is perceived as itch and induces more scratching. The
scratching impulse is beyond the control of the patient. Control of pruritus is important because mechanical injury
from scratching can induce release of pro inflammatory cytokine and chemokine,
leading to a vicious scratch-itch cycle perpetuating the AD skin rash. Since
classic antihistamines are ineffective in AD, it is assumed that this mediator
histamine does not have a crucial role in AD-related pruritus. In contrast,
stress-induced neuropeptides, proteases, kinins, and T cell derived cytokines
such as interleukin (IL)-31 are known to induce itch. IL-31 is strongly
pruritogenic and exerts its biologic activity through a receptor composed of
the IL-31 receptor A and oncostatin M receptor β protein, both of which are
over expressed in lesional skin of AD. These findings imply that IL-31 has an
important role in the pruritus of AD.
*May be the only manifestation of AD in adults.
†Not to be
confused with nummular eczema occurring outside the setting of AD.
Disease Course
AD has a broad
clinical spectrum that varies depending upon the age of the patient. It is divided into infantile, childhood and adolescent/adult
stages. In each stage, patients may develop acute, subacute and chronic eczematous
lesions. In all stages, pruritus is the hallmark feature and
often precedes the appearance of skin lesions; hence the concept that AD is
“the itch that rashes.”
Acute lesions predominate in infantile AD and are characterized by edematous,
erythematous papules and plaques that may exhibit vesiculation, oozing and
serous crusting. Subacute dermatitis is characterized by erythematous,
scaling papules and variable crusting. Chronic lesions, which typify adolescent/adult AD, present as (1) thickened plaques of skin, (2)
accentuated skin markings (lichenification), and (3) fibrotic papules (prurigo nodularis).
Perifollicular accentuation
(follicular eczema) and small, flat-topped papules (papular eczema) are
particularly common in individuals with darkly pigmented skin. In any stage of
AD, patients usually have dry, lackluster skin and the most severely affected
individuals may evolve to a generalized exfoliative erythroderma. All types of
AD lesions can leave post inflammatory hyper-, hypo- or (in more severe cases)
depigmentation upon resolution.
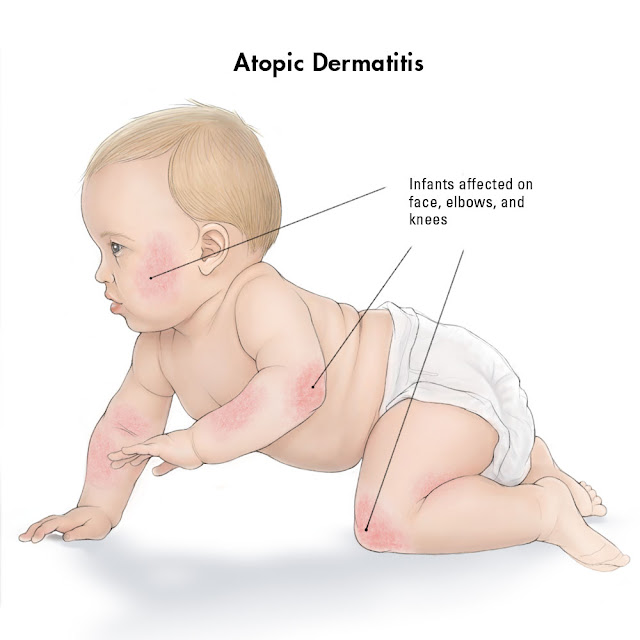
Infantile
AD (age 2 months to
2 years) typically develops after the second month of life. The
lesions most frequently start on the face, but may occur anywhere on the skin
surface, often initially appearing as erythema edematous papules and papulovesicles on the cheeks (often
sparing the central face), which may evolve to form large plaques with oozing
and crusting.
The face (especially around the mouth) and neck are affected in over 90 % of
infants. Secondary infection and lymphadenopathy are common. The eruption may extend to the scalp, neck, forehead, wrists, and
extensor aspects of the extremities and trunk
in
scattered, ill-defined, often symmetrical patches. Young infants may attempt to relieve itch through rubbing
movements against their bedding, whereas older infants are better able to
directly scratch affected areas. The areas involved
correlate with the capacity of the child to scratch or rub the site, and the
activities of the infant, such as crawling. By 8 to 10 months of
age, the exposed surfaces, especially the extensor aspect of the elbows and
knees often show dermatitis, perhaps
because of the role of friction associated with crawling and the exposure of
these sites to irritant and allergenic triggers such as those found in carpets.
Typically, lesions of AD spare the groin and diaper area during infancy, which
aids in the diagnosis. This sparing likely reflects the combination of
increased hydration in the diaper area, protection from triggers by the diaper,
and inaccessibility to scratching and rubbing. The disease runs a chronic,
fluctuating course, varying with such factors as teething, respiratory
infections, emotional upsets and climatic changes. Partial remission may occur during the summer, with relapse in winter.
This may relate to the therapeutic effects of ultraviolet (UV) B and humidity
in many atopic patients, and the aggravation by wool and dry air in the winter.
The infantile pattern of AD usually disappears by the end of the second year of
life.
Childhood AD (age 2 to 12 years), the childhood phase of AD usually occurs from 2 years of age to puberty. It resembles the infantile form early on, but later on evolves into features seen in the adulthood form. Affected persons in this age group are less likely to have exudative and crusted lesions and have a greater tendency toward chronicity and lichenification. Eruptions are characteristically drier and more papular and the erythematous and edematous papules tend to be replaced by indurated circumscribed scaly plaques. From 18 to 24 months onwards, the sites most characteristically involved are flexural areas (i.e., the antecubital and popliteal fossae, flexor wrists, sides of the neck, and front of ankles). These areas of repeated flexion and extension perspire with exertion. The act of perspiration stimulates burning and intense itching and initiates the itch-scratch cycle. Tight clothing that traps heat about the neck or extremities further aggravates the problem. Inflammation typically begins in one of the fossae or around the neck. The rash may remain localized to one or two areas or progress to involve the neck, antecubital and popliteal fossae, wrists, and ankles. The eruption begins with papules that rapidly coalesce into plaques, which become lichenified when scratched. The border may be sharp and well-defined, as it is in psoriasis, or poorly defined with papules extraneous to the lichenified areas. A few patients do not develop lichenification even with repeated scratching. The sides of the neck may show a striking reticulate pigmentation, sometimes referred to as ‘atopic dirty neck’. Facial involvement switches from cheeks and chin to periorbital and perioral, the latter sometimes manifesting as “lip-licker’s dermatitis”. Nail dystrophy may be seen when fingers are affected, indicating involvement of the nail matrix. AD often subsides as the patient grows older; leaving an adult with skin that is prone to itching and inflammation when exposed to exogenous irritants.
Severe AD
involving a large percentage of the body surface area can be associated with
growth retardation.
Adult/adolescent
AD (age >12 years) also features subacute to chronic,
lichenified lesions, and involvement of the flexural folds typically continues.
However, the clinical picture may also change. The adult phase of AD begins
near the onset of puberty. The reason for the resurgence of inflammation at
this time is not understood, but it may be related to hormonal changes or to
the stress of early adolescence. Most adolescents and
adults with AD will give a history of childhood disease and those patients who have suffered from continuous AD
since childhood are more likely to have extensive or even erythrodermic
disease.
One area or several
areas may be involved, and there are several characteristic patterns.
Inflammation
in flexural areas
This pattern
is commonly seen and is identical to childhood flexural inflammation.
Hand
dermatitis
Atopic
hand eczema affects
approximately 60% of adult patients with AD and may be the only manifestation
of the condition. Patients with AD often develop nonspecific, irritant hand
dermatitis.
Adults are exposed to
a variety of irritating chemicals such as harsh soaps, detergents, and
disinfectants in the home and at work, and they wash more frequently than do
children. It is extremely common for atopic hand dermatitis to
appear in young women after the birth of a child, when increased exposure to irritants
like soaps and water triggers their disease. Contact allergy may manifest as
chronic hand eczema as they are frequently exposed to preservatives and other
potential allergens in the creams and lotions that are continually applied to
their skin because of their dry skin.
Atopic hand
eczema typically involves one or both the volar wrists and dorsum of the
hands as well as palmar surfaces. Irritation causes redness and scaling on the
dorsal aspect of the hand or around the fingers. Itching develops, and the
inevitable scratching results in lichenification or oozing and crusting. A few
or all of the fingertip pads may be involved and become dry and peeling or red
and fissured. The eruption may be painful, chronic, and resistant to treatment.
The palms and sides of the fingers may develop the deep-seated vesicles of dyshidrotic
eczema. Involvement of the feet is also common and almost half the
patients with atopic hand eczema will have eczema on the feet.
Inflammation
around eyes
The lids are thin, frequently exposed to irritants, and easily traumatized by scratching. Many adults with AD have inflammation localized to the upper lids. In general, the involvement is bilateral and the condition flares with cold weather. Habitual rubbing of the inflamed lids with the back of the hand is typical. In contrast to eyelid eczema due to other causes, it is characterized by lichenification of the periorbital skin. As in hand dermatitis, irritants and allergic contact allergens must be excluded by a careful history and patch testing.
Lichenification
of the anogenital area
Lichenification
of the anogenital area is probably more common in patients with AD than in
others. Intertriginous areas that are warm and moist can become irritated and
itch. Lichenification of the vulva, scrotum, and perianal area may develop with
habitual scratching. These areas are resistant to treatment and inflammation
may last for years. The patient may delay visiting a physician because of
modesty, and the untreated lichenified plaques become very thick. Emotional
factors should also be considered with this isolated phenomenon.
Adults
frequently complain that flares of AD are triggered by acute emotional upsets
such as anxiety, and depression.
Even in
patients with AD in adolescence and adulthood, improvement usually occurs over
time, and dermatitis is uncommon after middle life. In general, these patients
retain mild stigmata of the disease, such as dry skin, easy skin irritation,
and itching and also susceptible to a flare of their disease when exposed to
the specific allergen and in response to heat and perspiration.
Senile AD (age >60
years) is
characterized by marked xerosis. Most of these patients do not have the
lichenified flexural lesions typical of AD in children and younger adults.
Regional Variants of Atopic Dermatitis
Face The
face is a frequent location for site-specific manifestations. Eczema of the
lips, referred to as cheilitis sicca, is common in AD patients,
especially during the winter. It is characterized by dryness (“chapping”) of
the vermilion lips, sometimes with peeling and fissuring, and may be associated
with angular cheilitis. Patients try to moisten their lips by licking, which in
turn may irritate the skin around the mouth, resulting in so-called lip-licker's
eczema. Frequently spreading some distance around the mouth, it
may become secondarily infected and crusted. Its persistence, and perhaps its
origin, is attributable to habits of lip licking, thumb sucking, dribbling or
chapping. It is easily transformed into true perioral dermatitis by the
application of potent corticosteroids. The regular application of 1%
hydrocortisone ointment is usually most helpful. Contact sensitivity, for
example to toothpaste ingredients, can occasionally be demonstrated.
Another common feature of childhood AD is ear eczema,
presenting as erythema, scaling and fissures under the earlobe and in the
retroauricular area, sometimes in association with bacterial superinfection and
this infra-auricular
fissures appears to be quite specific to atopic dermatitis.
“Head and neck dermatitis” represents a variant of AD that typically occurs after
puberty and primarily involves the face, scalp and neck. It is postulated that
lipophilic Malassezia yeasts, members of the normal skin flora that
colonize the head and neck area, represent an aggravating factor for this
condition. Serum levels of M. furfur-specific IgE have been shown to
correlate with the severity of head and neck dermatitis, and improvement with
systemic antifungal treatment has been observed in some patients.
Eczema variants also occur in acral sites.
Juvenile plantar dermatosis presents with “glazed” erythema, scale and fissuring on the
balls of the feet and plantar aspect of the toes in children with AD,
especially during the winter.
The prurigo form of AD favours the extensor
aspects of the extremities and is characterized by firm, dome-shaped papules
and nodules with central scale-crust, similar to prurigo nodularis lesions in
non-atopic patients.
Nummular lesions also tend to develop on the extremities in children and
adults with AD, appearing as coin-shaped eczematous plaques, usually 1 to
3 cm in diameter and often with prominent oozing and crusting (similar to nummular
dermatitis lesions in non-atopic patients). Colonization with Staphylococcus
aureus is thought to represent a trigger for this type of eczema. Intense
pruritus is a feature of both prurigo and nummular lesions. Frictional
lichenoid eruption has a predilection for atopic children (especially
boys) and presents as multiple small, flat-topped, pink to skin-colour papules
on the elbows and (less often) knees and dorsal hands. Lastly, localized
patches of atopic dermatitis can occur on the nipples, nipple eczema especially in
adolescent and young women.
The
pattern of atopic eczema varies with age. It may clear at any stage.
Associated features of
atopic dermatitis (“atopic stigmata”)
|
Xerosis |
|
||||||||||||||||||
|
Ichthyosis
vulgaris |
|
||||||||||||||||||
|
Keratosis
pilaris |
|
||||||||||||||||||
|
Palmar
and plantar hyperlinearity |
|
||||||||||||||||||
|
Dennie–Morgan
lines |
|
||||||||||||||||||
|
Periorbital
darkening (“allergic shiners”) |
|
||||||||||||||||||
|
Anterior
neck folds |
|
||||||||||||||||||
|
Hertoghe
sign |
|
||||||||||||||||||
|
White
dermographism |
|
||||||||||||||||||
|
Follicular
prominence |
|
||||||||||||||||||
Cutaneous stigmata
A linear
transverse fold just below the edge of the lower eyelids, known as the
Dennie–Morgan fold, is widely believed to be indicative of the atopic
diathesis, but may be seen with any chronic dermatitis of the lower lids. In
atopic patients with eyelid dermatitis, increased folds and darkening under the
eyes is common. When taken together with other clinical findings, they remain
helpful clinical signs. A prominent nasal crease may also be noted.
Dry
skin
The skin of atopic
individuals has been shown to be deficient in ceramides, filaggrin, and natural
moisturizing factors, leading to increased transepidermal water loss and
epidermal microfissuring. The less
involved skin of atopic patients is frequently dry, scaly and slightly
erythematous. Histologically, the apparently normal skin of atopics is
frequently inflamed subclinically and this dry, scaling skin of AD may
represent low grade dermatitis. Filaggrin is processed by caspase 14 during terminal
keratinocyte differentiation into highly hydroscopic pyrrolidone carboxylic
acid and urocanic acid, collectively known as the “natural moisturizing factor”
or NMF. Null mutations in FLG lead to reduction in NMF, which probably contributes
to the xerosis that is almost universal in AD. Transepidermal water loss (TEWL)
is increased through an abnormal stratum corneum, which
may also correlate with disease activity. This
may be caused by of abnormal ceramide and sphingosine
synthesis. The defective lipid bilayers
that result retain water poorly, leading to increased TEWL and clinical
xerosis.
Pityriasis alba
It frequently affects children and adolescents with AD. It
is characterized by multiple ill-defined hypo pigmented macules and patches
with fine scaling, which are typically located on the face (especially the cheeks)
but occasionally appear on the shoulders and arms. These lesions are most
obvious in individuals with darkly pigmented skin and/or following sun exposure.
Pityriasis alba is a low-grade eczematous dermatitis that disrupts the transfer
of melanosomes from melanocytes to keratinocytes. It usually responds
to emollients and mild topical steroids, preferably in an ointment base.
Keratosis
pilaris (KP)
Horny
follicular papules of the outer aspects of the upper arms, thighs, cheeks, and
buttocks, are commonly associated with AD. The keratotic papules on the face may
be on a red background, a variant of KP called keratosis pilaris rubra facei.
KP is often refractory to treatment. Moisturizers alone are only partially
beneficial. Some patients will respond to topical lactic acid or urea.
Thinning of
the lateral eyebrows, Hertoghe’s sign is sometimes present. This apparently
occurs from chronic rubbing due to pruritus and subclinical dermatitis.
Vascular stigmata
Atopic
individuals often exhibit perioral, perinasal, and periorbital pallor
(“headlight sign”). White dermatographism is blanching of the skin at the site
of stroking with a blunt instrument. This reaction differs from the triple
response of Lewis, in that it typically lacks a wheal, and the third response
(flaring) is replaced by blanching to produce a white line.
Atopics are at
increased risk of developing various forms of urticaria, including contact
urticaria. Episodes of contact urticaria may be followed by typical eczematous
lesions at the affected site.
Complications
Psychosocial
aspects
Atopic dermatitis has
a profound effect, equal to or greater than that of asthma and diabetes, on
many aspects of patients’ lives and the lives of their families. In children,
the most troublesome symptoms are itching, distress at bath time and difficulty
going to sleep. This can lead to behavioural difficulties and school
performance in severely affected children.
Infections
Infections/Agents associated with atopic dermatitis
Patients with AD are predisposed to the development of skin
infections because of factors including an impaired skin barrier and modified
immune milieu. Bacterial and viral infections represent the most common
complications of AD.
Bacterial
infections
Considering that S. aureus colonizes the skin of the
vast majority of patients with AD, it is not surprising that impetiginization
(which can also occur due to Streptococcus pyogenes) occurs quite
frequently. Bacterial infections may also exacerbate AD by stimulating the
inflammatory cascade, e.g. via S. aureus exotoxins that act as
super antigens. The importance of S. aureus colonization in AD is supported by the observation that
patients with severe AD, even those without overt infection, can show clinical
response to combined treatment with anti-staphylococcal antibiotics and topical
glucocorticoids. Methicillin-resistant S. aureus has become an increasingly
important pathogen in patients with AD.
Indeed, any acute
vesicular eruption in an atopic should suggest the diagnosis of secondary
bacterial or viral infection.
Viral
infections
Patients with atopic
dermatitis, both active and quiescent, are liable to develop acute generalized
infections with herpes simplex virus (eczema herpeticum), to produce the
clinical picture of Kaposi’s varicelliform eruption. Such episodes may present
as a severe systemic illness with high fever and a widespread eruption.
However, there may be no systemic disturbance, and at times the eruption may be
quite localized, often to areas of pre-existing atopic dermatitis.
After an
incubation period of 5-21 days, multiple, itchy, vesiculo-pustular lesions
erupt in a disseminated pattern; vesicular lesions are umbillicated, tend to
crop and often become hemorrhagic and crusted which result in numerous
monomorphic, extremely painful and punched-out erosions. These erosions may
coalesce to large, denuded, and bleeding areas that can extend over the entire
body. Eczema herpeticum is frequently widespread and may occur at any site,
with a predilection for the head, neck and trunk. Complications may include
superinfection with S. aureus or S. pyogenes as well as herpetic
keratoconjunctivitis and meningoencephalitis. Patients with mutations in the
filaggrin gene and those who have both severe AD and asthma have an increased
risk for eczema herpeticum, and decreased production of antimicrobial peptides
may have a pathogenic role. Patients with AD are also predisposed to the
development of widespread molluscum contagiosum, sometimes with
several hundred lesions. Individuals with AE have a higher incidence
of common warts.
Superficial fungal infection
Superficial
fungal infections are also more common in atopic individuals and may
contribute to the exacerbation of AD. Patients with AD have an increased
prevalence of Trichophyton rubrum infections compared to non atopic controls.
There has been particular interest in the role of M. sympodialis (Pityrosporum ovale
or P. orbiculare) in AD. M. sympodialis is lipophilic yeast commonly present in
the seborrheic areas of the skin. IgE antibodies against M. furfur are commonly
found in AD patients and most frequently in patients with head and neck
dermatitis. The potential importance of M. sympodialis as well as other
dermatophyte infections is further supported by the reduction of AD skin
severity in such patients after treatment with antifungal agents.
Ocular complications
Besides development of acute conjunctivitis as a component
of allergic rhinoconjunctivitis, the atopic eye disease also includes chronic
manifestations such as atopic keratoconjunctivitis (typically in adults) and
vernal keratoconjunctivitis (favors children living in warm climates). Atopic
keratoconjunctivitis is usually bilateral and symptoms include ocular itching,
burning, tearing and copious mucus discharge, often in association with
conjunctival injection and blepharitis that manifests as swelling and scaling
of the eyelids. Vernal keratoconjunctivitis is a severe bilateral recurrent
chronic inflammatory process associated with papillary hypertrophy or
cobblestoning on the upper palpebral conjunctiva. Keratoconusis an uncommon finding, occurring in approximately 1% of atopic
patients, is a conical deformity of the cornea
believed to result from chronic rubbing of the eyes in patients with atopic allergic
rhinoconjunctivitis. It is due to a degenerative change in the
cornea, which is forced outwards by the intraocular pressure, giving rise to
marked visual disturbances. Up to 10% of patients with AD
develop cataracts, either anterior or posterior, with anterior cataracts more specifically related to AD and
posterior cataracts occurring more commonly. Posterior
subcapsular cataracts in atopic individuals are indistinguishable from
corticosteroid-induced cataracts. Development of cataracts is more common in patients
with severe dermatitis and its peak incidence is between 15 and 25 years
of age. It is almost always bilateral. Cataract associated with AE is thought
to arise due to a combination of rubbing and the use of topical steroids.
Asthma and allergic rhinitis
Allergic rhinitis (hay fever) and asthma
occur in 30–50% of cases of AE. The age of onset is usually later than that of
the eczema. AE is a risk factor for the future development of allergic airways
disease, possibly by percutaneous sensitization to protein antigen through an
abnormal cutaneous barrier: the ’atopic march’.
Exfoliative Dermatitis
Patients with extensive skin involvement may develop exfoliative dermatitis. This is associated with generalized redness, scaling, weeping, crusting, lymphadenopathy, and fever. Although this complication is rare, it is potentially life threatening. It is usually due to superinfection, for example, with toxin-producing S. aureus or herpes simplex infection, continued irritation of the skin, or inappropriate therapy. In some cases, the withdrawal of systemic glucocorticoids used to control severe AD may be a precipitating factor for exfoliative erythroderma.
Diagnostic criteria
Major features in the
diagnostic criteria include pruritus, eczematous skin lesions in typical
age-specific distribution patterns, a chronic or chronically relapsing course,
early age at onset, and a personal and/or family history of atopy. Atopic
stigmata, especially xerosis, are also recognized as supporting features.
IgE-associated and non-IgE-associated AD are distinguished based on the
evaluation of total serum IgE levels (elevated in the former; normal
<150 IU/ml) and the presence or absence of specific IgE.
Histology
The histologic
features of AD depend upon the stage of the lesion sampled. Acute, exudative eczema is characterized by marked
spongiosis, with intraepidermal fluid collection leading to the formation of
vesicles (micro and macro) or even bullae. Some dermal edema may also be
present, together with perivascular lymphocytes that extend into the epidermis
and a variable number of eosinophils. In subacute lesions,
vesiculation is absent whereas acanthosis, hyperkeratosis, and parakeratosis
become evident. In chronic, lichenified
AD, epidermal thickening is more pronounced in a pattern that may be either
irregular or regular (psoriasiform). Changes in the granular layer vary from
thickening secondary to rubbing, as seen in lichen simplex chronicus, to
thinning when there is a psoriasiform pattern, seen in some nummular lesions.
Spongiosis and inflammation are less conspicuous, but there may be an increased
number of mast cells and dermal fibrosis.
These features are not specific,
as similar findings are observed in other eczematous dermatoses such as
allergic contact dermatitis. There are occasionally histologic clues to the
aetiology, such as individually necrotic keratinocytes that suggest an irritant
contact dermatitis. However, a skin biopsy is usually more helpful in excluding
other entities that can mimic AD clinically, such as mycosis fungoides.
Differential Diagnosis
In infants, AD is
often preceded and/or accompanied by seborrheic dermatitis, which commonly
presents during the first month of life as yellowish-white, adherent
scale-crusts on the scalp. Infantile seborrheic dermatitis also has a
predilection for skin folds (where lesions may be oozing and lack scale) and
the forehead, in contrast to the typical distribution of infantile AD on the
extensor surfaces of the extremities and cheeks as well as the scalp. Scabies
in infants often has generalized involvement and can mimic AD; in addition to
the presence of burrows or identification of the mite or eggs (e.g. via
dermoscopy or skin scrapings), scabies can usually be distinguished by the
predominance of discrete small crusted papules, involvement of the axillae and
diaper area, and the presence of acral vesiculopustules.
Adolescents
and adults without a personal or family history of atopy who present with an
eczematous eruption should have a thorough history and consideration of patch
testing to assess for allergic contact dermatitis. This diagnosis should also
be considered in children and adults with established AD who fail to respond as
expected to treatment or who develop lesions in an atypical distribution
pattern. Components of emollients or topical corticosteroid preparations
represent potential allergens in these individuals. Protein contact dermatitis
has a predilection for atopic individuals and can also present as a chronic
eczematous dermatitis. Causes include a variety of foods and animal products,
and it is diagnosed by prick testing or observation of an urticarial reaction
within 30 minutes of patch testing on previously affected skin.
Laboratory testing is not needed in the routine
evaluation and treatment of uncomplicated AD. Estimation of total serum IgE,
specific radio allergosorbent tests (RASTs) and prick tests usually serve only
to confirm the atopic nature of the individual.
Serum IgE levels are
elevated in approximately 70–80% of AD patients. This is associated with
sensitization against inhalant and food allergens and/or concomitant allergic
rhinitis and asthma. In contrast, 20–30% of AD patients have normal total IgE
levels and negative RASTs, whereas 15% of apparently healthy individuals have a
raised serum IgE levels. This subtype of AD has a lack of IgE sensitization
against inhalant or food allergens. It may be that skin prick test positivity
to food allergens in young children with severe atopic dermatitis and a high
serum IgE indicates a high risk of developing later allergic respiratory
disease. The majority of patients with AD also have peripheral blood
eosinophilia.
Prognosis
The following predictive factors correlate
with a poor prognosis for AD: widespread AD in childhood associated allergic
rhinitis and asthma, family history of AD in parents or siblings, early age at
onset of AD, being an only child, and children with raised IgE antibodies to foods
and inhalant antigens at 2 years of age.
Measuring the severity of atopic
dermatitis
The severity
of dermatitis can be measured and monitored in several ways. The SCORing Atopic
Dermatitis (SCORAD) index, the Objective Severity Assessment of Atopic
Dermatitis (OSAAD) and the Three Item Severity Scoreall have merit in a
research context, but are not practical for daily clinical use.
The severity
assessment has been simplified with the objective to stratify treatment
accordingly in individual patients.
- Measuring the area involved in
percentage of body surface, where 1% body surface approximates the size of
one of the patient's hands (including the fingers).
- Establishing acute, subacute or
chronic changes, where acute changes would imply more severe dermatitis.
- Determining the impact on the
patient's quality of life (e.g. sleep disturbances, absenteeism, visible
scratch marks and social withdrawal).
Dermatitis
can then be classified and treated as mild, moderate or severe as outlined
below.
Treatment
Because
AD is a chronic relapsing disease, the classic approach to therapy is targeting
acute flares with short-term treatment regimens, i.e. reactive management.
Based on recent insights into the underlying skin barrier defect and its
relationship to inflammatory processes in the skin and other organs, a
proactive approach that includes long-term maintenance therapy is now recommended.
This treatment strategy may modify the overall disease course and possibly
prevent the development of atopic comorbidities. Management of AD includes
education of patients/parents, gentle skin care, moisturizer use, and
anti-inflammatory therapy to control subclinical inflammation as well as overt
flares. Topical agents represent the mainstay of treatment. Severe disease may require
phototherapy or systemic medications, usually in conjunction with continued
topical therapy. Factors that can potentially exacerbate AD including
irritants, relevant allergens and microbial agents should be
identified and, if possible, avoided.
B. Intermittent courses of a systemic corticosteroid result
in rebound flares and worsening of disease over time. In contrast, a proactive
regimen utilizing topical corticosteroid leads to longer clear periods and
milder disease over time. TCI, topical calcineurin inhibitor.
General care
Education (patients and parents)
Patient and parent education is effective in the
management of AD and should aim to provide information about the clinical
characteristics of AD (aetiology, clinical manifestations, and disease course
in common person language), aggravating and relieving factors, self-management
and improving coping skills. Parents often seek an eradicable
cause for their child’s AD and have difficulty accepting “control” rather than
a “cure” for the condition.
Education of patient or caregiver is highly
recommended at each consultation in the management of AD and should encompass:
a. appropriate treatment doses and application frequency; b. how to step up or
step down treatment; c. skin care and bathing; d. management of infection. This
leads to more effective management of AD and should be reinforced at every
consultation.
Bathing
Bathing and showering are important not only for
hydration of the skin and for washing away the components of perspiration but also for
washing away allergens, such as, dust and pollens, scale,
irritants and microbes on the
skin surface. Bathing also allows removal of dirt and debris from the skin and
thereby reduces the chance of infection. Swimming should be avoided in acute
flares as the amount of free residual chlorine may impact the skin barrier and
contribute to AD exacerbation.
It is
generally recommended that patients bath or shower once daily with lukewarm
water (27 degree Celsius to 30 degree Celsius), which is not too hot and not
too cold, for a short period of time (e.g., 5 to 10 min). Although allowing
moisture to fully evaporate from the skin following bathing can worsen xerosis,
application of an emollient to the skin within 3 minutes of exiting a daily
lukewarm bath increases skin hydration and barrier function. If treatment with
a topical corticosteroid or other anti-inflammatory agent is needed, it should
be applied immediately after bathing, prior to the moisturizer. For acute
flares of AD, 10- to 20-minute soaks in lukewarm or tap water compresses
followed directly with corticosteroid application (“soak and smear”) this has
been referred to as the “soak and smear” technique.
Bubble
baths and scented oils should be avoided. Scalp care should include a bland
shampoo.
Cleansing
The skin must be cleansed thoroughly, but gently
and carefully to get rid of crusts and bacterial contaminants in case of
bacterial super infection. Strong scrubbing or rubbing immediately after bath
should be avoided. Skin should be dried using soft towels.
Use of non-soap cleansers (e.g., Syndet) that
are neutral to low pH, hypoallergenic, non-irritant and fragrance free is
recommended for AD.
Moisturizers/
emollients
As there
is impaired skin barrier in the pathogenesis of AD, daily use of moisturizers
constitutes the core of the management of AD. Continuous basic therapy with
emollients is also required even in periods and sites in which the AD is not
active. It should be part of all
treatment phases; mild, moderate, and severe. A
moisturizer repairs the skin barrier, maintains skin integrity and appearance,
reduces transepidermal water loss, and restores the lipid barrier's ability to
attract, hold, and redistribute water; thereby it can reduce xerosis, pruritus,
erythema, fissuring, and lichenification. They make topical
corticosteroids more effective and reduce the quantities required. The regular use of moisturizer has short and
long term steroid-sparing effects in mild to moderate AD and in preventing AD
flares.
Standard
moisturizers contain varying amounts of emollient agents that lubricate the
skin, occlusive agents that prevent water loss, and humectants that attract
water. Preparations should be free of dyes, fragrances, food-derived allergens
such as peanut protein, and other potentially sensitizing ingredients such as
perfumes, lanolin and herbal extracts. The formulation of the emollient should
be chosen based upon the degree of dryness of the skin, the sites of
application, acceptance by the patient and the season. Ointments (e.g.
petrolatum) contain higher concentrations of lipids, have occlusive properties,
and are typically preservative-free; although they tend to cause less stinging
when applied to inflamed skin. However, the greasiness of an ointment is not
acceptable to all patients. Creams may be a more acceptable option for such
individuals, whereas lotions contain higher water content and are not ideal for
the xerosis of AD. The addition of moisturizing factors that is able to bind
water (e.g. glycerol, urea) leads to increased hydration of the epidermis;
although with higher concentrations of urea or α-/β-hydroxy acids, which can decrease scaling, may sting
when used in children and on acutely inflamed or excoriated skin.
Emollients should be applied twice daily to the entire cutaneous
surface.
Persistent
use of emollients when the inflammation is in remission may reduce the time to
and frequency of flares.
In order to identify the emollient that best suits an
individual, it may be useful to provide small quantities of several agents, so
that they may choose which they prefer. Although no
clinical trials have studied the proper amount or frequency of moisturizer use
in patients with AD, moisturizer should be used at least twice daily and more
frequently during acute flare-ups. A generous quantity (150–250 g/week
for young children and 500 g/week for older children and adults) should be
prescribed to encourage their frequent use throughout the day. It is recommended to use moisturizer within 3 min after
taking a bath while the skin is still moist.
Prescription emollient devices
(PEDs) that aim to improve the defective skin barrier of AD include
preparations that contain specific ratios of lipids (e.g. cholesterol, fatty
acids, and ceramides), palmitoylethanolamide, glycyrrhetinic acid, and other
hydrolipids. There is currently no evidence that these agents are superior to
over-the-counter preparations.
STEPLADDER
TREATMENT IN ATOPIC DERMATITIS
Depending on the severity of the AD, topical
treatment methods and/or systemic treatments are recommended. It is recommended
to implement stepladder treatment appropriate to the clinical severity. Once
remission is achieved, it is advisable to shift to proactive maintenance
therapy to reduce the number of subsequent flare ups.
Topical corticosteroids
Topical
corticosteroids represent first-line pharmacologic therapy for AD who has
failed good skin care, including moisturizer use. These agents have
anti-inflammatory, antiproliferative, immunosuppressive, and vasoconstrictive
actions, with effects on cutaneous T cells, macrophages, and dendritic
cells. Topical corticosteroids are used
to treat acute flares of AD and as maintenance therapy to prevent relapse.
Efficacy: RCTs have demonstrated safety and
continued efficacy of repeated courses of low- to mid-potency TCS on active AD
skin until clearance for up to 5 years in children and up to 1 year in adults.
Long-term
proactive TCS: The proactive approach of applying low–mid potency TCS twice
weekly for the prevention of flares in stabilized AD has been shown to be
effective in both adults and children.
Long-term TCS adverse effects: When used cautiously, long-term use of low
to mid potency TCS is reasonably safe. However, the long term use of TCS,
especially if high potency may cause local side effects, such as, striae
rubrae, skin atrophy, telangiectasia, skin burning, erythema and acneiform
eruptions. In rare cases, systemic effects may occur including
hypothalamic–pituitary–adrenal axis suppression, more frequently in children
due to the high ratio of total body surface area to body mass, which is about
2.5 to 3 times higher than for adults. The use of twice-weekly proactive
treatment has not been shown to cause skin atrophy.
Frequency of
application: Twice daily application of TCSs is generally recommended for
the treatment of AD; however, evidence suggests that once
daily treatment in the evening, with morning application of emollients, may be
as effective as twice daily corticosteroid treatment. Proactive, intermittent
use of TCSs as maintenance therapy (one to two times per week) on areas that
commonly flare is recommended to help prevent relapses and is more effective
than use of emollients alone.
Topical
corticosteroids designed to have decreased systemic bioavailability and a favourable
therapeutic index (e.g. fluticasone propionate, mometasone furoate) may be
preferable, especially for infants and young children with widespread
involvement.
Factors in
selecting the potency and vehicle of the topical corticosteroid include the
location, type (e.g. acute versus chronic), thickness and extent of the AD
lesions; patient age and preference as well as the cost and availability of
different preparations represent additional considerations. The corticosteroid
should have an appropriate potency to quickly gain control of the flare, and
continuation of daily therapy until the active dermatitis is completely clear
minimizes the likelihood of a rebound. Long-term daily use of an inadequately
potent topical corticosteroid can result in a greater risk of side effects (as
well as less control of the eczema) than relatively brief use of a more potent
agent. After clinical resolution of longstanding or severe lesions, tapering to
every-other-day treatment may be considered prior to decreasing to maintenance
therapy. For children and adults with moderate to severe AD, the risk of
relapse can be significantly reduced by proactive maintenance with twice-weekly
application of a mid-potency topical corticosteroid to the usual areas of
involvement (“hotspots”) when clear (in conjunction with emollient
use), with no evidence of cutaneous atrophy after up to a year of treatment.
For the
face and body folds, high-potency corticosteroids should be avoided if possible
due to risk of cutaneous atrophy and (for the face) acneiform eruptions.
However, short-term use of a potent agent (e.g. mometasone furoate ointment)
may be required to clear thick, exuberant lesions on the cheeks of infants. Potent
corticosteroids (e.g. class 1–2) are often needed for thick or lichenified
plaques, nummular or prurigo-like lesions, and eczema on the palms and soles.
Flurandrenolide tape represents another option for prurigo-like lesions, since
it physically blocks scratching and rubbing of the affected area.
Corticosteroid ointments (which minimize burning/stinging) are generally
preferred considering the dryness of the skin in AD patients and the emollient
effects of these vehicles. Application immediately after bathing improves
cutaneous penetration and also decreases burning. Corticosteroid solutions or
foams represent choices for AD on the scalp. Corticosteroid‐resistant
or infected or crusted dermatitis may respond better to steroid/antibiotic
combinations.
Systematic
reviews have concluded that topical corticosteroids have a favorable safety
profile with short-term (up to several weeks) daily use and long-term
intermittent use. However, “steroid phobia” is very common among AD patients
and their parents, and it often leads to delayed and inadequate treatment. It
is essential that these fears and incorrect beliefs regarding topical
corticosteroids are addressed to ensure adherence to the treatment plan.
When AD
does not respond as expected to topical corticosteroid therapy, adherence
should be assessed, including the amount (grams/tubes) and duration
(consecutive days) of use. If possible, in-patient therapy for patients with
severe AD can allow direct observation and intensive education. Potential
complicating factors should be investigated, such as disease exacerbation by super
infection, irritants, or allergens. The latter can include immediate
hypersensitivity reactions to foods and aeroallergens as well as delayed
hypersensitivity to contact allergens, including components of moisturizers and
topical mediations.
Monitoring
corticosteroid use
Topical steroids can cause side effects if abused. It is
advisable to educate patients about the quantities to apply, for example the adult fingertip unit. A strip of ointment measured
from the distal phalangeal crease to the tip of the finger, or approximately 0.5 g, being
applied over an area equal to two adult palms, following the rule of 9's that
measures the percent of affected area and use of charts that propose amounts
based on patient age and body site. It would seem prudent to monitor the
height and weight in young children if they have severe dermatitis requiring
moderately potent or potent steroids; however, any effects on growth are more
likely to arise from active dermatitis rather than the treatment. Local side
effects, such as permanent telangiectasis on the cheeks in babies and striae of
the breasts, abdomen and thighs in adolescents, may be minimized if appropriate
steroid strengths are used. Particular care is required around the eyes, as
glaucoma may be induced by topical steroids. When there are concerns about side
effects, the topical calcineurin inhibitors, pimecrolimus and tacrolimus, may
prove to be helpful.
Topical
calcineurin inhibitors
TCIs can be considered as first-line therapy along with
appropriate use of moisturizing agents. Two topical
calcineurin inhibitors (TCIs) have been approved by the US Food and Drug
Administration (FDA) for the treatment of AD: tacrolimus 0.03% and 0.1%
ointment (for “moderate to severe” disease) and pimecrolimus 1% cream (for
“mild to moderate” disease) not
controlled by topical corticosteroids. These
agents suppress T-cell activation and modulate the secretion of cytokines and other
pro inflammatory mediators; they also decrease mast cell and dendritic cell
activity. Their efficacy in the treatment of AD has been proven in
adults and children ≥2 years of age (and, for pimecrolimus, infants ages 3–23
months, although it is not approved for this group). Both agents have been shown to be effective in
short-term (3–12 weeks) and long-term (up to 12 months) studies in adults and
children with active disease.
A
meta-analysis of 25 RCTs found tacrolimus 0.1% to be as effective as the
mid-potency TCS hydrocortisone butyrate 0.1%, whereas tacrolimus 0.03% is less
effective than hydrocortisone butyrate 0.1% but more effective than the
low-potency TCS hydrocortisone acetate 1%.
TCIs are
particularly suitable for AD affecting the face and intertriginous areas, sites
where corticosteroid-induced skin atrophy is of increased concern and TCI
therapy is especially effective. TCIs are also beneficial in patients with
frequently flaring or persistent AD that would otherwise require almost
continual use of topical corticosteroids. Recent randomized controlled studies
have shown that application of tacrolimus ointment twice weekly (weekend therapy) to “hotspots” as a proactive
management during maintenance can prevent flares of AD without
increasing the overall amount of medication used and has been shown to be effective and safe for up
to 1 year in both children and adults.
Generally,
worldwide they are perceived as second line agents, and their attraction is the
absence of the cutaneous side effects of skin atrophy, striae, telangiectasia
and bruising that may be seen with prolonged or inappropriate corticosteroid
use.
TCIs are very safe, the most common side effect
being skin burning on initial
application. This sensation often settles with
continued use in 80% of patients after 1 week
or if TCI use is preceded by a short topical corticosteroid course and so it may be worth introducing to a limited area
initially to gain patient confidence. TCIs do not
increase the risk of bacterial infections, but the risk of viral infections
such as herpes simplex virus is slightly elevated. There was a boxed warning
based on a theoretical risk of malignancy against TCI since 2006. However,
there is no convincing evidence, either from controlled studies with follow-up
of patients or from studies of patient databases that TCIs can induce malignant
disease. The largest and longest trial looking at infants treated with
pimecrolimus found no evidence of increased malignancy risk.
They
would seem to be of value for resistant eczema, particularly around the eyes
and in areas at high risk of skin thinning and also for maintenance therapy.
Oral
corticosteroids
Of note,
no systemic medications besides corticosteroids have been FDA-approved for
treatment of AD. However, in general, treatment of AD with systemic
corticosteroids should be avoided due to a propensity for significant
rebound flares upon their discontinuation and the unacceptable side effects of
long-term use. In the occasional exception of a severe, generalized acute flare
(e.g. with a specific trigger) resistant to aggressive topical management,
treatment with a systemic corticosteroid should be limited to a short course
(up to 1 week) with transition to a topical regimen,
phototherapy and/or an alternative systemic agent. Long-term use of oral
corticosteroids in AE patients is not recommended.
Oral prednisolone (typically 0.5
mg/kg) is used in the management of severe exacerbations of AE. Generally, oral
corticosteroids are well tolerated and act rapidly, which can be highly valued
by patients suffering from severe flares. Unfortunately, because there is usually
disease recurrence following cessation of treatment, the clinical benefit
achieved by oral corticosteroids in those patients who do not apply concomitant
intense topical therapy may lead to patient preference for intermittent courses
of oral corticosteroids and disengagement with topical treatment.
Second Line of Therapy
Ciclosporin
Cyclosporine (CsA) is an oral calcineurin
inhibitor that suppresses the activation of the T-cell transcription factor,
nuclear factor of activated T cells, inhibiting the transcription of a number
of cytokines, including IL-2.
Oral ciclosporin is the first
choice among systemic immunomodulators in both adults and children older than 2 years of age who are unresponsive to
conventional topical treatment or showing severe course of disease. However, the use of oral cyclosporine to treat AD is
limited by potential side effects such as nephrotoxicity (which can develop
after as little as 3–6 months of therapy) and increased blood pressure, which
seem to be dose-dependent. The duration of cyclosporine therapy is
guided by clinical efficacy and tolerance of the drug. Both short-term and
long-term therapies may be useful in AE. The drug should be administered twice a day at a
daily dose of 3–5 mg/kg/day. Low starting doses (3 mg/kg/day) and high starting
doses (5 mg/kg/day) are found to be equally effective after 2 weeks. Once
clinical efficacy is reached, a gradual dose
reduction of 0.5– 1.0 mg/kg/day every 2 weeks is recommended, until a minimal effective maintenance dose (usually
~2 mg/kg/day).
Virtually all patients respond rapidly with a reduction in eczema severity by
approximately 55% at 6–8 weeks. However, symptoms recur rapidly when drug
therapy is discontinued. Both continuous long-term
(up to 1 year) and intermittent short-term dosing schedules (3- to 6-month
courses) are efficacious. Longer term use of
ciclosporin is associated with an increased side effect profile. However, if
the dose can be reduced down to or below 2.5 mg/kg and regular monitoring of
renal function and blood pressure are satisfactory, ciclosporin may be
continued for up to 1 year.
Of note,
cyclosporine is often not sufficient as monotherapy, requiring combination with
topical corticosteroids to reach an almost complete remission.
Since an intermittent-dosage regimen (e.g.
‘weekend therapy’) will lead to lower cumulative doses of cyclosporine and is
effective in some AE patients, an individualized dosage regimen is recommended
for children and adolescence patients.
Although there are no
controlled studies available regarding the efficacy of vaccination during
cyclosporine therapy, there is no evidence for a failure during cyclosporine
either. Hence, a cessation of therapy of 2 weeks before and 4–6 weeks after
vaccination may be advisable. Clinically, there is no evidence for this
recommendation.
Third Line of Therapy
Phototherapy
Phototherapy can be one
of useful treatment modalities for moderate to severe AD. Currently, narrow‐band phototherapy seems to be the preferred option for
chronic disease for both adults and children. On average, disease activity is
reduced by approximately 30–50% at the end of a typical 24‐treatment
course. Moreover, improvement may be maintained for several months. It can be used as monotherapy or in combination with
emollients and topical steroids. Acute flares of AE should be treated with
intense treatment prior to narrow‐band UVB phototherapy and secondary infection should also be
treated. In general, phototherapy courses should be limited to one per annum.
In young children, phototherapy may be difficult for practical reasons, e.g.
lack of cooperation.
Azathioprine
Azathioprine should be considered as a
second-line choice among systemic immunomodulators in adult patients
unresponsive to or experiencing side effects with cyclosporine.
Azathioprine has a slow onset of
action than
ciclosporin, with clinical improvement after
1–2 months and full benefit requiring 2–3 months of treatment. Clinical improvement may be
maintained for several months after discontinuation of azathioprine therapy.
The
dosage of azathioprine should be adjusted according to patient's ability to
metabolize the drug as determined by the activity of the enzyme thiopurine methyltransferase
(TPMT) measured in red blood cells as individuals with
genetically determined low activity of the enzyme thiopurine methyltransferase
(TPMT) have increased susceptibility to azathioprine-induced myelotoxicity. Patients with absent TPMT activity should not receive
azathioprine; those with normal or high TPMT activity should receive azathioprine
at a dose of 1–3 mg/kg/day, and those with low TPMT activity should receive
azathioprine at a dose of 0.5–1 mg/kg/day. Measurement of red blood cell
thioguanine nucleotide (the active metabolites of azathioprine) levels is now
available as a routine assay and may be useful for titrating azathioprine dose,
particularly in patients with low TPMT levels. Regular blood monitoring is
still required for patients receiving azathioprine as TMPT polymorphisms
account for only 65% of azathioprine‐induced neutropenias. It is also important to bear in mind
the potential for drug interactions with common prescribed drugs such as
allopurinol.
Nausea, vomiting and
other gastrointestinal (GI) symptoms are common while on AZA. The other side
effects include headache, hypersensitivity reactions, elevated liver enzymes
and leukopenia.
Methotrexate
MTX seems to be a well-tolerated and effective third-line
option for the long-term treatment of moderate-to-severe AD in both children
(above 8 years) and adults.
Methotrexate can be considered as a second-line
choice among systemic immunomodulators after cyclosporine.
Methotrexate has anti-inflammatory effects and reduces
allergen-specific T-cell activity. It can have efficacy for refractory AD in
adults and children with weekly administration of 7.5–25 mg or 0.3–0.5 mg/kg,
respectively, together with folic acid supplementation. This regimen is well tolerated,
with maximum clinical effect typically seen after 2–3 months of therapy. Methotrexate appears equi‐efficacious as azathioprine. Also,
and similar to azathioprine, methotrexate may result in persistent improvement
for several months after discontinuation. Clinical experience suggests that it
may be better tolerated than azathioprine. As MTX is
teratogenic, men and women of childbearing potential must use effective
contraception during therapy.
Myclophenolate
mofetil
Mycophenolate mofetil (MMF) inhibits the de novo pathway of purine synthesis, resulting in
suppression of lymphocyte function. It may be of benefit for recalcitrant AD in
adults and children who have
failed to respond to or are intolerant of azathioprine and/or methotrexate.
Dosing generally ranges from 1 to 3 g/day in adults and 30–50 mg/kg/day in
children, with 2–3 months of treatment typically required for
maximum effect.
MMF is generally well tolerated, with GI
symptoms being the most commonly encountered. Hematologic (anaemia, leukopenia,
thrombocytopenia) and genitourinary (urgency, frequency, dysuria) symptoms are
rarely reported.
As
MMF and EC-MPS are both teratogenic, men and women of childbearing potential
must use effective contraception during therapy.
In
summary, the approach for systemic therapy for moderate to severe AE includes
the use of short duration of therapy of ciclosporin to gain control of acute
flares.
For patients with resistant moderate
to severe AE with chronic and more stable disease both azathioprine and
methotrexate may be considered as first line options. For patients with chronic
resistant disease unresponsive or intolerant of azathioprine and methotrexate,
myclophenolate mofetil is used.
Fourth Line of
Therapy: Biologics and Emerging Therapies
Crisaborole
Crisaborole ointment 2% is a topical
phosphodiesterase-4 inhibitor approved by FDA for the treatment
of mild to moderate AD in patient ≥2 years of age. PDE-4 degrades cAMP, which leads to increased
production of pro inflammatory cytokines
including IL-10 and IL-4, thereby reduces disease severity and pruritus severity.
The most common side effect is stinging or burning in the area of application.
Dupilumab
Dupilumab is a fully human monoclonal antibody
directed against the IL-4a receptor a-subunit, which blocks the signaling of
both IL-4 and IL-13, the two key drivers of Th 2 immune response. In 2017,
dupilumab was approved by the US Food and Drug Administration (FDA) for
treatment of moderate to severe AD in patients 18 years of age and older
that is not adequately controlled with topical therapy and other systemic
treatment is not advisable. A
systematic review and meta-analysis of efficacy and safety of dupilumab
treatment in moderate to severe AD provided evidence that dupilumab had an
acceptable safety profile and resulted in clinically relevant improvements in
signs and symptoms of AD.
Dupilumab is administered via
subcutaneous injection of 600 mg initially and then 300 mg every other week. Dupilumab
should be combined with daily emollients and may be combined with topical
anti-inflammatory drugs as needed. It has a
favourable side effect profile, with injection site reactions and
conjunctivitis each occurring in ~10% of patients.
Apremilast
Apremilast, an oral phosphodiesterase-4
inhibitor, was FDA-approved in September 2014 for the treatment of
moderate-to-severe plaque psoriasis. However, its upstream anti-inflammatory effect,
ease of use as an oral agent, and mild side effect profile make it an interesting
treatment option for AD as well. Multiple open-labeled trials of apremilast in
moderate-to-severe AD produce conflicting reports on efficacy, some showing
fair and other showing limited efficacy.
In patients with recalcitrant atopic dermatitis,
biologics especially dupilumab or phosphodiesterase-4 inhibitor like apremilast
can be used as off-label therapy. However, the cost effectiveness should be
seriously considered.
Maintenance therapy
The proactive use of topical anti-inflammatory
therapy to address subclinical inflammation is an effective, contemporary
clinical strategy for the management of AD. Proactive treatment with TCS and
TCI are effective to prevent AD flares.
The
continuous use of emollients should be emphasized, as often in mild disease
prolonged control can be obtained with the addition of very intermittent use of
topical corticosteroids. In more problematic patients, the addition of twice
weekly application of potent topical steroids to the healed areas (the
’weekender approach’) can safely and significantly reduce relapses of AE. If
relapse occurs, topical corticosteroids should be used daily again for a week
and then stepped down to the weekender approach once more.
The topical calcineurin inhibitors
have also been used to maintain control applying tacrolimus or pimecrolimus
twice weekly. Adding mid‐week use of tacrolimus ointment to the topical
corticosteroid weekender approach has been used to improve control of severe AE.
Adjunctive Therapy
Antimicrobials and antiseptics
Because S.
aureus, which densely colonizes the skin in most AD patients, is known to
amplify the cutaneous inflammation that underlies AD, reduction of bacterial
load can play a role in the management of AD.
0.005% sodium hypochlorite baths (0.5 cup of household bleach [6% sodium
hypochlorite] added to a full 40-gallon bathtub) twice weekly together with a
monthly 5-day course of intranasal topical mupirocin ointment for 3-months led
to greater improvement of moderate to severe, superinfected AD. Cleansers and
emollients containing antiseptics such as triclosan or clioquinol represent
additional options. In general, the use of topical and systemic antibiotics
should be restricted to short-term treatment of super infections in order to
prevent the development of bacterial resistance. There is some evidence that
the S. aureus strains that colonize and superinfect patients with AD are
more likely to be susceptible to first-generation cephalosporins (e.g.
cephalexin).
Although
90% of patients with AE are colonized with S. aureus,
the routine use of systemic antibiotics for AD is not recommended. However,
systemic antibiotics can be utilized when AD patients display clinical evidence
of bacterial infection caused by
staphylococcal and sometimes streptococcal infection such as
pustules, a purulent exudate, or furuncles. In patients with recurrent flares of AE associated with
infection, prolonged antibiotic treatment, topical steroid/antibiotic
combination creams and measures to reduce staphylococcal colonization of the
nose and perineum, could be considered.
Parents
should be advised about the risk of herpes simplex infection in a child with
AE, and told to avoid contact of active cold sores with the child's skin. Eczema herpeticum due to herpes simplex infection should be
treated without delay with oral aciclovir. If the patient is febrile or toxic,
intravenous therapy should be considered.
VZV vaccination is recommended for children
with atopic dermatitis. Parents of atopic children should be encouraged to
fully immunize their children.
Topical or systemic antifungal therapy may be
effective in some AE patients, mainly in those suffering from the ‘head and
neck’ variant of AE or with demonstrated IgE sensitization to Malassezia spp.
AE
is not a contraindication to routine childhood vaccinations. Although egg protein
is present in some vaccines, the amount is so small that it should not be a
problem in practice, unless the child has documented severe systemic reactions
to egg protein; if in doubt, vaccination should be supervised in an environment
where resuscitation equipment is available.
Itch
and antihistamines
Itch
is the most difficult symptom of AE to treat, and currently there is no
specific antipruritic treatment; it seems controlling inflammation is the most
effective treatment. The use of anti‐inflammatory preparations, emollients and reduction of
trigger factors remains the initial approach.
The usefulness of oral antihistamines is controversial and
debated. Published data from randomized controlled
trials (RCTs) are available for both sedating and non sedating antihistamines;
the results of these trials generally suggest a limited role for antihistamines
in the treatment of AD.
H1‐receptor antagonists are used
predominantly for their sedative effect and can be
useful in breaking the “itch–scratch cycle”. Agents such as hydroxyzine, promethazine or trimeprazine
given 1 h before bedtime can be useful when there is severe nocturnal itching
with significant sleep disturbance.
However, they can cause drowsiness and lack of concentration the next morning.
In infants, these preparations may occasionally cause paradoxical excitation.
They are best used in short courses, early in treatment until topical treatment
is effective. Most studies conclude that non‐sedating antihistamines are of
little value for the pruritus of AE but in some cases with an allergic
component, for example contact urticarial or hay fever, they may be of value. Second generation antihistamines (cetirizine) may be used
short-term, under supervision where itch of eczema causes sleep disturbance
especially in children under age of 2 years. Cetirizine also shows
steroid-sparing effect. Addressing itch in young atopics is of primary
importance. The nocturnal itch that accompanies AD leads to a fall in QOL
indices. The active scratching can further disturb the skin barrier function.
This will worsen the atopic state.
Wet
wrap technique
Wet wrap therapy (WWT) can be helpful to quickly
reduce AD severity, and it is often useful for acute flares and/or recalcitrant
disease. These moist, occlusive
dressings increase skin hydration, act as a barrier to scratching, and enhance
the penetration of topical corticosteroids. Two layers of
absorbent tubular bandage are applied to the skin. The inner layer is
pre-soaked in warm water and the outer layer is dry cotton gauze or garments. A
generous quantity of a diluted low‐potency
topical corticosteroid is applied to the skin before the bandage; they are left
in place overnight or changed every 12 h and the
treatment duration should not exceed 2 weeks. This regimen can be used in hospital or for short‐term
out‐patient treatment. Close supervision should be maintained
because suppression of the hypothalamopituitary axis can occur when topical
steroids are employed. Regimens using emollient only under the wet dressings
have become popular but are somewhat less effective.
It is suggested that its use might not be
feasible in every region of India due to varied climatic conditions. However,
wherever suitable, it can be used in severe or resistant patients older than 6
months of age.
Vitamin D
RCTs have reported contradictory results for the
therapeutic value of vitamin D supplementation in AD. One meta-analysis showed
that serum vitamin D level was lower in the patients with AD, and vitamin D
supplementation could be a new therapeutic option for AD. Vitamin D has a
potentially significant role for improving the symptoms of AD. The results from
this study suggest that vitamin D supplementation may help ameliorate the
severity of AD and can be considered as a safe and tolerable therapy.
Controlling Factors Responsible for Exacerbation of AD
Trigger factors
Multiple
environmental and psychological factors can trigger AD, including allergens
(e.g. pollen, dust mites, animal dander), sweating, harsh soaps, wool or other
rough fabrics, cigarette smoke and emotional stress. These vary depending upon
the patient and may be identified (and subsequently avoided) by a careful
history and allergy testing. Fluorescence enzyme immunoassays (which have
largely replaced RAST tests) can be performed on serum samples to quantify
specific IgE antibodies against suspected allergens; skin prick testing
represents another option to assess for immediate hypersensitivity. The atopy
patch test can provoke an eczematous reaction through the epicutaneous
application of aeroallergens or food allergens, with readings at 48 and 72 hours
to detect delayed hypersensitivity. It holds promise as it is a specific tool
to evaluate children with AD for clinically significant allergies.
Spectrum of trigger factors at
different ages
Most
patients have dry skin, and soaps and detergents can irritate the dermatitis.
Many patients will suffer winter exacerbations. Simple measures such as turning
down the central heating and not heating the bedroom may make life more
comfortable.
Various
allergens associated with atopic dermatitis
Food allergens
There are conflicting evidences available for
associations of specific food with AD.
Food allergens may contribute to eczema. However, empirical food
restriction is NOT recommended in patients of atopic dermatitis. The food
should be restricted based only on clinical experience and food diagnosis
procedures. A specific-food-free diet (especially for infants or toddlers)
should only be considered when allergy to the specific food trigger is
identified based on proper procedures, including a food diary and allergy test
by a specialist.
Approximately
30% of infants and young children (especially younger than 7 years) with AD with moderate to severe AD who is unresponsive to routine therapy have food allergy. Food allergy in older children and adults is rare. ~90% of reactions in this population are
caused by five allergens: eggs (most often linked to AD exacerbations), milk and milk products, peanuts,
soy, and wheat. Exposure to food
allergens may exacerbate eczema in ~10–30% of infants and young children with
AD, especially those with severe, recalcitrant disease. However, food allergens
more often produce an immediate/IgE-mediated reaction with urticaria, flushing,
or itch within 1–2 hours of exposure.
The National Institute of Allergy and Infectious Diseases (NIAID)
recommends food allergy testing in children <5 years of age with moderate to
severe AD if they also have: (1) persistent AD activity despite optimized
management; or (2) a reliable history of an immediate allergic reaction after
ingestion of a specific food.
Testing
1.
Perform a skin-prick test.
2.
Determine specific IgE antibodies. Sera are
analyzed for total IgE and specific IgE antibody titres (e.g., to cow’s milk,
hen’s egg, wheat, and soy).
Allergen-specific IgE assays and skin prick tests are not reliable.
The clinical history and (in selected instances) provocation tests should be
used to determine the relevance of
positive laboratory and skin prick tests, since these allergens may not
necessarily be exacerbating the patient’s AD. When relevant food allergens are
identified and avoided, skin-directed AD therapy is still crucial. A trial of
an exclusion diet can be recommended for foods that produce positive skin-prick
tests or are thought to be a possible cause.
It is
important that parents and/or patients understand that coexistent food
allergies are not the “cause” of AD. Even in patients with a clinically
relevant allergy, elimination diets can prevent immediate hypersensitivity but
are less likely to affect the course of the AD.
Clothing
Smooth clothing and avoidance of irritating
fabrics and fibres and loose-fitting garments are recommended in patients with
AD. Use of woollen, acrylic and nylon fabric should be avoided. Cotton is
recommended as best fabric.
Sweating
Sweating is an important exacerbating factor for
AD, hence washing away sweat by bathing and showering will lead to the
improvement of symptoms. Avoiding occupational or recreational exposure to high
temperature and humidity can help in controlling exacerbation.
Aeroallergen reactivity
Aeroallergen
reactivity increases with age and is more prevalent in those with moderate to
severe AD. Common aeroallergens include dust mites, pollens, animal dander, and
fungi. Exacerbation by aeroallergens should be considered when AD is more
severe in exposed areas, and direct skin contact with aeroallergens may trigger
the development of eczematous lesions in some patients. Evaluation includes
assessment of specific IgE antibodies and skin prick testing.
Among all aeroallergens, house‐dust
mite allergen appears to be the most important. Avoid
house‐dust allergens in the home
by dust‐mite eradication measures
(sealed containment bags for the mattress, pillows and bedding top covers, a
high‐power vacuum cleaner and a miticide sprays) result in significant reductions in
dust‐mite antigen (Der p 1) load
in carpets and beds and is associated with great clinical benefits in both
adults and children (over 6 years old) with severe AE.
Animal
dander can aggravate AE and contribute to house‐dust
mite antigen levels, and so the keeping of household pets should be
discouraged. Spring and summer flare, often in association with hay fever, can
be related to exposure to grass and tree pollens. This pattern is often
associated with a facial distribution in the older child.
Allergic contact dermatitis
Patch
testing to assess for contact sensitivity should be considered in AD patients
with findings suggestive of ACD, a distribution pattern atypical of AD, sudden
worsening, or recalcitrance to treatment. Common contact allergens in AD
patients include components of topical medications and skin care products, such
as fragrance, preservatives, lanolin, propylene glycol, cocamidopropyl betaine,
bacitracin, neomycin, and sometimes corticosteroids.
Psychological factors and psychosomatic interventions
Patients with AD often
complain aggravation due to stress. Minimizing stress may be helpful in
controlling the disease. Psychotherapeutic approaches and behaviour therapy can
be considered to manage individual emotional factors that trigger AD, such as, vicious
itch-scratch cycles, co morbidity with anxiety and depression, and low quality
of life (QOL). Not only can stress aggravate AE, but the
severely affected child is also a source of stress to the whole family.
Prevention
Although
the therapeutic armamentarium available for AD can successfully control the
disease in most patients, primary prevention of AD represents a highly
desirable goal. There is no evidence that maternal food allergen avoidance
during pregnancy or lactation protects against the development of AD in the
child. Recent studies have found that low maternal prenatal 25(OH) vitamin D
levels may be associated with an increased risk of early-onset AD in the
infant. For infants with a family history of atopy, exclusive breastfeeding during
the first 4 months of life or feeding with a formula containing hydrolyzed milk
products may potentially decrease the risk of AD development compared to
feeding with a formula containing intact cow’s milk protein. However, exclusive
breastfeeding for 6 months versus 4 months does not confer additional
protection against the development of AD.
Probiotics/prebiotics
In
several randomized controlled studies, administration of probiotics (e.g. Lactobacilli) or prebiotics, which represent
non-digestible oligosaccharides that promote the growth of desirable bacteria,
to pregnant mothers and infants was associated with significantly decreased
frequencies of AD at 1 to 4 years of age. However, multiple randomized
controlled studies have failed to show benefit from probiotics as therapy for
existing AD. Further investigation is required to determine what pro/prebiotic
agent should be employed and the optimal time of administration for AD
prevention.
Emollient
therapy as prevention
Skin
barrier impairment, measured by an increase in transepidermal water loss,
occurs before the onset of clinical AD in children with FLG mutations. Two randomized controlled trials in
neonates with a family history of AD showed that daily use of anemollient
beginning within the first 3 weeks of life resulted in a 30–50% reduction in
the likelihood of developing AD by 6–8 months of age.
Emollients,
either creams or ointments, improve barrier function by supplying the stratum
corneum with water and lipids. Cetaphil cream—an oil-in-water petrolatum-based
cream—is an example of a commonly used product. Studies have shown that
Cetaphil cream improves skin barrier function. Apply the emollient once daily
or more often. The cream is most effective when applied within 3 minutes after
bathing. Minimize soap exposure during bathing. Use a fragrance-free mild
cleanser.
CONCLUSION
· The
prevention of allergic disease and allergies remain a key focus.
· Exclusive
breastfeeding for the first 4 months of life appears to decrease the incidence
of atopic disease.
· The
itch-and-scratch cycle of AD exacerbates symptoms, and breaking this cycle is
critical in managing this chronic disease.
· Treatment
should be individualized according to patient characteristics and disease
severity.
· A
combination approach may be used to calm the flare with a topical
corticosteroid for several days, followed by initiation of a topical
calcineurin inhibitor.
· Topical
calcineurin inhibitors are particularly useful in treating the face and other
thin, sensitive areas of the skin.
· Patients
should be advised about the potential side effects of selected pharmacologic
treatment, as compliance increases when patients know what to expect with
treatment.
Considerations in pediatric populations
The
principle of AD treatment is similar between adult and pediatric populations.
TCSs
Different TCSs preparations
with respective age limits have been approved by the FDA. Given the higher ratio
of body surface area to weight in children than in adults, less potent TCSs
should be used. A systematic review reported less than 3% of mild adrenal
suppression in children treated with TCSs and suggested close clinical
monitoring of this very rare phenomenon is adequate. The monthly dosages
of TCSs are in the average of 15 g for infants, 30 g for children,
and up to 90 g for adolescents (and adults). As children are more
susceptible to steroid-induced glaucoma and cataract, long-term use of TCSs
around the eyelids and periorbital regions should be avoided in children with
AD.
TCIs
Both tacrolimus 0.03% ointment
and pimecrolimus 0.1% cream are licensed for patients aged ≥2 years as
second-line therapy for acute and maintenance phases. However, they can be used
off-label in those <2 years, including in infants, as supported by clinical
trial evidence. Tacrolimus 0.1% ointment is indicated in adults and
adolescents aged 16 years and above. TCIs may be useful in children requiring
long-term topical treatment or frequent use of mild TCS for facial AD.
Antihistamines
Although sedating
antihistamines are used for sleep disturbance due to pruritus, it is not
recommended for long-term use because they may affect children's quality of
sleep. A recent non-interventional study found that early use of
antihistamines in children with AD was associated with increased symptoms of
attention-deficit/hyperactivity disorder (ADHD). However,
the relationships among AD severity, sleeping problems, antihistamine use, and
mental health problems are complex, and no adverse effects on behavior or
learning processes were found in infants with AD using the second-generation
antihistamine cetirizine.
Phototherapy
Phototherapy is not
contraindicated in children, but it is less used than in adults. In
general, the use of phototherapy in young children should be cautious as
long-term safety remains unknown. NB-UVB has a good safety profile in children;
however, caution must be exercised when considering other modalities, as their
safety is less certain.
Systemic
immunomodulatory agents
Although systemic treatments
may be used in pediatric AD, less clinical data are available. The use of
oral corticosteroid in pediatric population should be limited and even more
cautious than in adults due to well-known side effects and high incidence of
rebound flare upon discontinuation. Some agents have different starting
doses in children than in adults, e.g., 10–15 mg/m2/week for
methotrexate, and 20–50 mg/kg/day for mycophenolate
mofetil. Dupilumab has been approved for patients aged ≥12 years in the US
in March 2019.
Others
Wet wrap is a useful technique
along with other topical therapies, but is labor-intensive and not commonly
practiced. Also, evidence on the efficacy of wet wrap over standard care
is of low-quality. Wet wrap may enhance systemic absorption of TCSs/TCIs
and potential side effects deserve attention. Sleep disturbance is common
in children with AD, and clinical trials suggested melatonin can safely improve
disease severity and sleep quality. Mental health disorders (e.g.,
anxiety, depression, ADHD, etc.) are co morbidities in children and adolescents
with AD and may be exacerbated by poor sleep and disease
severity. Recently, involvement of pediatric psychologists in a
personalized integrative multidisciplinary program has been shown valuable for
difficult-to-treat pediatric AD, and psychological factors predicted long-term
treatment success. It is important for physicians to recognize these risks
and provide necessary referrals for psychotherapeutic interventions.
Considerations during pregnancy and lactation
Women with AD who required
treatment during pregnancy and lactation deserve special
consideration. Physicians should note the prescribing information of each
modality and exercise good clinical judgement when treating these patients.
TCSs
Overall, the use of TCSs in
pregnancy, with the exception of fluticasone propionate, has been considered
safe. Fluticasone propionate should be avoided because it is not metabolized by
the placenta. Avoid using very potent TCSs since they may be associated with
low birth weight, although rescue therapy for localized chronic/lichenified
lesions may be acceptable. TCSs should be applied after breastfeeding, and the
nipples should be cleaned before feeding.
TCIs
There are limited data on the
use of TCIs during pregnancy and lactation. Tacrolimus ointments should be used
only if clearly needed during pregnancy and are not recommended during
lactation despite low systemic absorption; pimecrolimus should be used with
caution during pregnancy and should not be used on the breast during lactation.
However, based on experience from oral calcineurin inhibitors and low systemic
absorption of TCIs, along with favorable benefit/risk ratio of TCIs, off-label
use of TCIs during pregnancy is recommended. Similar to TCSs, TCIs should be
applied after breastfeeding, and the nipples should be cleaned before feeding.
Antihistamines
With their limited clinical
efficacy, antihistamines may be used if indicated during pregnancy. Cetirizine
and loratadine both have a good safety profile in pregnant women, and
loratadine is preferred because of the large clinical experience. Physicians
should use sedating antihistamines with strict indications and weigh the risk
and benefit carefully.
Phototherapy
Both NB-UVB and UVA1 are
recommended when clinically feasible, but psoralens should be avoided. UVB
therapy decreases folic acid levels, and thus it should be supplemented
preconceptually and during the first trimester. Care should be taken for
pregnancy-induced hyper pigmentation (e.g., melasma) as phototherapy may
exacerbate this condition.
Systemic
immunomodulatory agents
The use of systemic
corticosteroids as rescue therapy should be limited for short-term (<2–3
weeks) and up to 0.5 mg/kg/day during pregnancy and lactation, with
prednisolone preferred over dexamethasone in pregnant patients. Cyclosporine
may be used after careful evaluation as first-line systemic therapy for
long-term control during pregnancy and lactation. The use of azathioprine is
debated and generally not recommended, but it may be used if other alternatives
are not available. Methotrexate and mycophenolate mofetil are absolutely
contraindicated during pregnancy and lactation, and they must be discontinued
for a period of up to 6 months prior to conception. Dupilumab is not
recommended during pregnancy and lactation until more data and experience are
available.