Systemic lupus erythematosus
Introduction
Systemic lupus erythematosus (SLE) is a systemic autoimmune
disease, with multi systemic involvement. The disease has several phenotypes,
with varying clinical presentations in patients ranging from mild mucocutaneous
manifestations to multi organ and severe central nervous system involvement.
Several immunopathogenic pathways play a role in the development of SLE. The
lupus erythematosus (LE cell) was described by Hargraves in 1948. Several
pathogenic auto antibodies have since been identified. Despite recent advances
in technology and understanding of the pathological basis and risk factors for
SLE, the exact pathogenesis of SLE is still not well known. Diagnosis of SLE
can be challenging and while several classification criteria have been posed,
their utility in the clinical setting is still a matter of debate.
The
improvement in patient survival – 50% four year survival in 1950; 85% 15 year
survival in 2019 – is probably due to multiple factors, including earlier
disease recognition with more sensitive diagnostic tests and improved
treatment.
Management of SLE should be individualized according to
predominant symptoms, organ involvement, response to previous therapy and
disease activity and severity.
Despite the improvement in outcome,
patients with SLE still have mortality rates two to five times higher than the
general population.
Clinical and Immunologic Criteria Used in the Systemic Lupus
International Collaborating Clinics (SLICC) Classification System*2012
Classify a
patient as having SLE if he or she satisfies four of the clinical and
immunologic criteria, including at least one clinical criterion and one
immunologic criterion, or if he or she has biopsy-proven nephritis
compatible with SLE in the presence of ANAs or anti-dsDNA antibodies. The criteria are cumulative and do not need
to be present concurrently.
Clinical criteria
1.
Acute
cutaneous lupus, including:
1.
Lupus
malar rash (do not count if malar discoid)
2.
Bullous
lupus
3.
Toxic
epidermal necrolysis variant of SLE
4.
Maculopapular
lupus rash
5.
Photosensitive
lupus rash in the absence of dermatomyositis
OR
Subacute cutaneous lupus (non-indurated psoriasiform
and/or annular polycyclic lesions that resolve without scarring, although
occasionally with post inflammatory dyspigmentation or telangiectasias)
2.
Chronic
cutaneous lupus, including:
1.
Classic
discoid rash
·
Localized
(above the neck)
·
Generalized
(above and below the neck)
2.
Hypertrophic
(verrucous) lupus
3.
Lupus
panniculitis (profundus)
4.
Mucosal
lupus
5.
Lupus
erythematosus tumidus
6.
Chilblain
lupus
7.
Discoid
lupus/lichen planus overlap
3.
Oral
ulcers:
Palate, buccal, tongue OR nasal ulcers in the
absence of other causes, such as vasculitis, Behçet’s disease, infection
(herpesvirus), inflammatory bowel disease, reactive arthritis, and acidic foods
4.
Nonscarring
alopecia (diffuse thinning or hair fragility with visible broken hairs) in the
absence of other causes such as alopecia areata, drugs, iron deficiency, and
androgenic alopecia
5.
Synovitis
involving 2 or more joints, characterized by swelling or effusion
OR tenderness in 2 or more joints and at least
30 minutes of morning stiffness
6.
Serositis:
· Typical pleurisy for
more than 1 day duration OR pleural effusions OR pleural rub
· Typical pericardial
pain (pain with recumbency improved by sitting forward) for more than 1 day
duration OR pericardial effusions OR pericardial rub OR pericarditis by
electrocardiography in the absence of other causes, such as infection, uremia
and Dressler pericarditis
7.
Renal:
1.
Persistent
proteinuria >500 mg of protein/24 hours
2.
or red blood cell casts
8.
Neurologic:
Seizures; psychosis; mononeuritis multiplex in the
absence of other known causes such as primary vasculitis; myelitis; peripheral
or cranial neuropathy in the absence of other known causes such as primary
vasculitis, infection and diabetes mellitus; or acute confusional state in the
absence of other causes, including toxic/metabolic, uremia, drugs.
9.
Hemolytic
anemia
10.
Leukopenia
(<4000/mm3 at least once) in the absence of other known causes
such as Felty syndrome, drugs and portal hypertension OR
Lymphopenia (<1000/mm3 at least once) in
the absence of other known causes such as corticosteroids, drugs, and infection
11.
Thrombocytopenia
(<100,000/mm3) at least once in the absence of other known causes
such as drugs, portal hypertension, and thrombotic thrombocytopenic purpura
Immunologic criteria
1.
ANA
level above laboratory reference range
2.
Anti-dsDNA
antibody level above laboratory reference range (or >2-fold the reference
range if tested by ELISA)
3.
Anti-Sm:
presence of antibody to Sm nuclear antigen
4.
Antiphospholipid
antibody positivity as determined by any of the following:
·
Positive
test result for lupus anticoagulant
·
False-positive
test result for rapid plasma reagin
·
Medium-
or high-titer anticardiolipin antibody level (IgA, IgG, or IgM)
·
Positive
test result for anti–β2-glycoprotein I
(IgA, IgG, or IgM)
5.
Low
complement:
·
Low
C3
·
Low
C4
·
Low
CH50
6.
Direct
Coombs’ test in the absence of hemolytic anemia
Epidemiology
Varying prevalence and incidence rates of SLE have been reported,
with differences mostly attributes to the population differences.
The strongest factor affecting risk for lupus is gender. SLE predominantly affects women of childbearing
age, with female to male ratio of 9 to 1; it is likely that hormonal
factors influence susceptibility. The risk, however, decreases after menopause
in women although still is twice as compared to men. Studies have indicated
that although rare, lupus in men tends to be more severe. Although SLE is much
more common in female patients, its diagnosis, treatment and management remains
the same for male patients.
Age
plays an important role in SLE, and although the disease is more common in
childbearing age in women, it has been well reported in the pediatric and
elderly population. The condition tends to occur in early adult
life, and the peak age of onset of the first symptom or sign in females is
approximately 38 years. SLE is more severe in children while in the elderly, it tends to
be more insidious onset and has more pulmonary involvement and serositis and
less Raynaud's, malar rash, nephritis, and neuropsychiatric complications.
Etiology
SLE is a multi factorial disease with unknown exact etiology;
however, several genetic, immunological, endocrine, and environmental factors
play a role in the etiopathogenesis of SLE.
Genetic factors
Familial segregation and high concordance rates in identical twins
suggest a strong genetic contribution in SLE, although there is no obvious
pattern of inheritance. Concordant rates for identical twins have been reported
to be as high as 50%. More than 50 genes or genomic loci have been
identified to be associated with SLE, most encoding proteins implicated in the
function of the immune system. These genes are associated with activation
of the immune system in response to foreign antigens, self-antigen generation,
and activation of innate and adaptive immune systems. Some gene mutations that
are rare, but are considered very high risk for the development of SLE
include deficiencies of early complement components C1q, C1r, C1s (>90%
risk), C4 (50%), C2 (20%) and TREX1. One of the chromosome regions having the
strongest association with SLE is the human leucocyte antigen (HLA) locus,
especially the class region containing HLA‐DRB1, ‐DQA1
and ‐DQB1. Women
are at 10 times more risk of developing SLE than men, and the risk of SLE is 14
times more in Klinefelter syndrome (47, XXY). This suggests an association
with genes on the X-chromosome, however, despite several studies, the exact
genes have not been identified.
Immunological factors
Interactions
between susceptibility genes, hormonal influences (90% of SLE patients are
female) and environmental factors result in abnormal immune responses,
resulting in autoantibody production and consequent dysregulation of the
inflammatory response, leading to induction and maintenance of the disease.
Autoantibodies may be present for a several years before the first clinical
symptom appears.
Endocrine factors
Female sex and hormonal influence is a significant risk factor for
SLE. Estrogens and prolactin promote autoimmunity and increase the B-cell
activation factor production and modulate lymphocyte and pDC activation. The
use of estrogen-containing contraceptives and postmenopausal hormone
replacement therapy can cause flares in patients with SLE and have been associated
with a higher incidence of SLE. Elevated levels of prolactin are seen in
patients with SLE. Androgens, on the other hand, are considered
protective.
Environmental Factors
Genetic predisposition for a lupus diathesis does not, in
itself, produce disease. Rather, it appears that induction of autoimmunity in
such patients is triggered by some inciting event, likely an environmental
exposure. Drugs, viruses, UV light, and, possibly, tobacco, have been shown to
induce development of SLE.
Ultraviolet radiation
Ultraviolet radiation
(UVR) is probably the most important environmental factor in the induction
phase of SLE and especially of LE-specific skin disease. UV radiation may
precipitate the onset or exacerbate the course of SLE in up to 60% of patients. UV
light likely leads to self-immunity and loss of tolerance because
it causes apoptosis of keratinocytes, which in turn, makes previously
cryptic peptides available for immunosurveillance. UVB radiation has been shown
to displace autoantigens such as Ro/SS-A and related autoantigens, La/SS-B, and
calreticulin, from their normal locations inside epidermal keratinocytes to the
cell surface. UVB irradiation induces the release of CCL27 (cutaneous T
cell-attracting chemokine), which upregulates the expression of chemokines that
activate autoreactive T cells and interferon-α (IFN-α), producing dendritic
cells (DCs), which likely play a central role in lupus pathogenesis.
Tobacco smoking
Smoking is also thought
to be a risk, with a dose-response. Smokers are at a greater risk of developing
SLE than are nonsmokers and former smokers (lipogenic aromatic amines).
Patients with treatment resistant CLE are much more likely to smoke. Several
authors have shown that patients with LE-specific skin disease who smoke are
less responsive to antimalarial treatment.
Drugs
Several drugs have been
implicated in causing a lupus-like phenomenon by causing demethylation of DNA
and alteration of self-antigens. While procainamide and hydralazine have
the highest incidence of causing drug-induced lupus, more than 100 drugs have
been associated with drug-induced lupus. Further, several drugs such
as the sulfa-drugs are well known to cause flares in patients with SLE.A range
of medications can induce systemic LE (SLE) and cutaneous LE, most commonly
subacute cutaneous LE (SCLE). In the
former, patients may develop fever, malaise, polyarthritis, and serositis from
drugs such as hydralazine and procainamide, but associated acute cutaneous LE
is rare. Although symptoms typically appear at least one month after drug
initiation and resolve days to weeks after discontinuation of the responsible
medication, the skin lesions of SCLE are sometimes more persistent.
The pathogenesis of drug-induced SLE is not well understood, but
one possibility is that reactive drug metabolites, interacting with nuclear
histones, could act as haptens and activate the complement cascade. For
example, procainamide-induced SLE occurs more frequently in patients who are
slow acetylators as compared to rapid acetylators.
The precipitation of SLE by drugs, especially hydralazine and procainamide is well known. However, there are features to suggest that drug‐induced SLE differs from spontaneous disease: it occurs in an older age group, renal and central nervous system involvement are infrequent, antihistone antibodies are frequent, antibodies against double-stranded DNA are typically absent and serum complement is normal.
While drug-induced SLE is characterized by the presence of antihistone antibodies in up to 95% of cases, these antibodies are not specific and may be seen in patients with idiopathic SLE. The seroconversion from negativity to positivity for antinuclear antibodies alone is not sufficient to discontinue a particular medication, but if symptoms develop, the offending drug should be withdrawn. However, the antinuclear antibodies may persist for 6 to 12 months.
Cutaneous involvement in drug‐induced SLE may be vasculitic, bullous or erythema multiforme‐like or resemble pyoderma gangrenosum. Minocycline‐induced SLE is uncommon, it usually occurs after 2 years of therapy. Patients who require more than 1 year's therapy should have ANA and liver function tests monitored. Other drugs, particularly certain anticonvulsants, are known to precipitate SLE‐like syndromes.
In drug-induced SCLE, anti-SSA/Ro and anti-SSB/La antibodies are often present and the cutaneous and histologic findings are indistinguishable from those seen in the idiopathic form of the disease. It therefore behooves the clinician to carefully review all medications in patients with the diagnosis of SCLE, in particular terbinafine, thiazide diuretics, proton pump inhibitors, calcium channel blockers, and taxanes. Of note, patients receiving TNF-α inhibitors may develop cutaneous lesions of chronic (discoid), subacute, or acute cutaneous LE as well as antinuclear and anti-DNA antibodies. In these patients, arthritis often predominates over cutaneous manifestations and some patients tolerate a switch to a different TNF inhibitor.
There is an increased
incidence of HLA-DR4 in drug-induced SLE and the ratio of females to males is
4: 1, indicating a possible genetic predisposition. It appears that individuals
who are slow acetylators are more likely to develop drug- induced LE or LE-like
syndromes. The determination of acetylator type and DR typing may enable
susceptible patients to be identified.
Viruses
There has been much
speculation about the role of infectious agents, particularly viruses, in the
induction of SLE and CLE. Seroconversion to Epstein-Barr virus (EBV) among
patients with SLE is nearly universal, and recent data have demonstrated that
patients with SLE have defective control of latent EBV infection that probably
stems from altered T-cell responses against EBV. Antibodies against
Epstein-Barr virus (EBV) are more prevalent in children and adults with SLE
compared to the general population.
Other potential risk factors include silica exposure, other viral
infections, vitamin D deficiency, alfalfa sprouts and foods containing
canavanine.
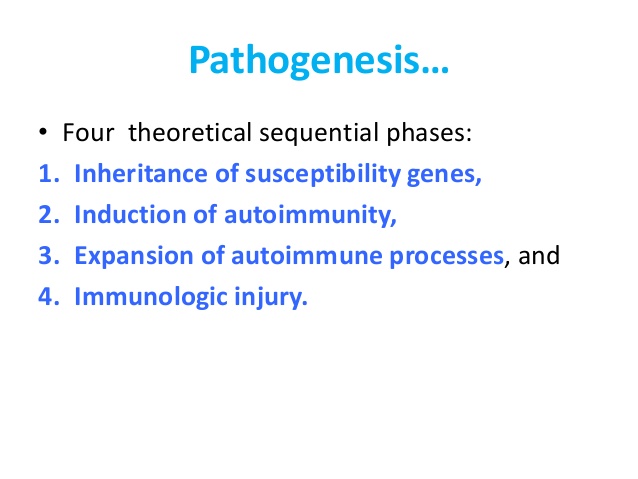
Pathogenesis
SLE is a disorder in which the interplay
between host factors (susceptibility genes, hormonal milieu, etc.) and
environmental factors [ultraviolet (UV) radiation, viruses, and drugs] leads to
loss of self-tolerance, and induction of autoimmunity. This is followed by
activation and expansion of the immune system, and eventuates in immunologic
injury to end organs and clinical expression of disease.
The pathogenesis of SLE is complex, and the understanding of SLE
pathogenesis is constantly evolving. A break in the tolerance in genetically
susceptible individuals, on exposure to environmental factors, leads to the
activation of autoimmunity. Cell damage caused by infectious and other
environmental factors exposes the immune system to self-antigens leading to
activation of T and B cells which become self-sustained by a chronic self-aimed
immune response. Cytokine release, complement activation, and autoantibody
production then lead to organ damage.
Both innate and adaptive immune systems play a role in the
pathogenesis of SLE. The innate immune system activation is either Toll-like
receptor (TLR) dependent, or independent. The cell membrane-bound TLRs (TLR 2,
4, 6) are activated on exposure to the extracellular DNA and RNA from dying
cells, which leads to downstream activation of the Interferon regulatory family
(IRF-3), NF-κB and MAP-kinases, which serve as transcription factors for the
production of pro inflammatory mediators such as IFN-b. The endosomal TLRs (TLR
7, 9) are activated by single-stranded RNA and demethylated DNA and lead to the
production of Interferon-alpha, and RNA binding autoantibodies such as
antibodies against Ro, La, Sm, and RNP. The TLR-independent pathway
is activated by intracytoplasmic RNA sensors (RIG-1, MDA-5) and DNA sensors
(IFI16, DAI) and leads to activation of IRF3 and NF-κB. Both self DNA/RNA
and foreign DNA/RNA such as from viruses can lead to this
activation. NETosis has recently gained attention in the pathogenesis of
SLE. Neutrophils, on activation by various factors such as cytokines, activated
platelets and vascular endothelial cells systematically release their nuclear
aggregates in the extracellular environment. These nuclear aggregates can then
promote Interferon-alpha production by the dendritic cells, mediate thrombosis
and vascular damage and serve as self-antigens for T-lymphocytes.
T-lymphocytes and B-lymphocytes play a significant role in the
pathogenesis of SLE. Apoptotic and damaged cell-derived antigens are presented
to T-cells by antigen-presenting cells. T-cells in SLE display a distorted gene
expression leading to the production of several cytokines. These T-cells
produce less IL-2, which leads to altered regulatory T-cell production.
Increased IL-6, IL-10, IL-12 and IL-23 increases mononuclear cell production
while increased IL-17 and IL-21 leads to increased T-cell production. Increased
Interferon-γ leads to defective T-cell production. T-cells lead to the
activation of auto reactive B-cells by CD40L and cytokine production, which
leads to autoantibody production, which is a hallmark of SLE. Toll-like
receptors on interaction with DNA and RNA lead to activation of these B-cells,
and the nucleic acid and protein-containing intranuclear complexes are the most
prominent antigens leading to B-cell activation. These auto antibodies are
pathogenic and cause organ damage by immune complex deposition, complement, and
neutrophil activation, and altering cell function leading to apoptosis and
cytokine production. Further, the auto reactive B-cells in SLE which are
stimulated by self-antigens, are not readily eliminated due to a
deficiency of the process involved in the functional neutralization of auto reactive
B cells. The B-cells can also serve as antigen-presenting cells and can
activate T-cells by presenting internalized soluble antigens to T-cells. This
creates a loop where both B and T cells activate each other, both leading to
more autoimmunity.
IMMUNOLOGY OF SLE
The
production of type I interferon (IFN) by plasmacytoid dendritic cells (pDCs) is
a common mechanism of pathogenesis in SLE. Dying tissue cells release nucleic
acids; these form large complexes with antimicrobial peptides, such as LL37 and
with endogenous DNA-binding proteins, such as high mobility group protein B1
(HMGB1). These DNA and RNA complexes activate pDCs via Toll-like receptor 9
(TLR9) and TLR7, respectively, and they induce the production of type I IFN. In
turn, type I IFN promotes T cell activation, autoantibody production by B cells
and the release of neutrophil extracellular traps (NETs) that consist of
DNA–antimicrobial peptide complexes. Autoreactive antibodies activate neutrophils
and form DNA-containing immune complexes that are preferentially endocytosed by
pDCs via Fc receptors. Self-nucleic acids also activate classical DCs (cDCs)
and they promote the release of inflammatory cytokines and the priming of T
cells that are specific for self-antigens in a process that is also facilitated
by type I IFN.
Histopathology
Tissue pathology is SLE can demonstrate a variety of aberrant
immunologic mechanisms including immune complex formation, autoantibody
formation, and immunologically mediated tissue injury.
LE body or hematoxylin body is a hallmark of SLE
pathology. It is a homogeneous globular mass of nuclear material that
stains bluish-purple with hematoxylin. It can be observed in the lungs,
kidneys, spleen, heart, lymph nodes, and serous and synovial membranes. They
contain immunoglobulins as well as DNA, and engulfment of LE body by phagocytes
leads to the formation of the classic LE-cell.
Pathology from skin lesions in SLE demonstrates immune complex
formation leading to tissue damage, vascular and perivascular inflammation and
chronic mononuclear cell infiltration. Acute lesions demonstrate fibrinoid
necrosis at the dermo-epidermal junction and the dermis along with liquefactive
degeneration of the epidermis and perivascular inflammatory cell infiltration
with T-cell predominance. Chronic lesions can also
demonstrate hyperkeratosis and follicular plugging. Edema and RBC
extravasation can be seen in all SLE lesions. Immunofluorescence demonstrates
deposition of IgG, IgA, and IgM immunoglobulins and complement
components along the dermal-epidermal junction.
Vasculitis is common in SLE and vascular lesions in SLE may
demonstrate various different pathologies. Immune complex deposition with
an inflammatory response is the most common lesion, although it may be seen
without significant inflammatory response as well. Small and large vessel
necrotizing vasculitis with fibrinoid necrosis is less common but can be seen
and can be differentiated from other vasculitides by immune complex deposition
in the vessel wall. Thrombotic microangiopathy can be seen in patients with SLE
and antiphospholipid antibody syndrome.
Central nervous system pathology in SLE reveals small intracranial
vessel involvement with thrombotic lesions with or without perivascular
inflammation and endothelial proliferation. Necrotizing vasculitis can be seen
rarely. Thromboembolism from Libman-Sacks endocarditis has been seen as
well.
Cardiac pathology may include valvular involvement leading
to Libman-Sacks endocarditis which is sterile verrucous endocarditis.
It tends to involve the mitral valve most commonly with vegetations seen on the
forward flow side of the valve. Pathology reveals platelet thrombi, necrotic
cell debris, proteinaceous deposits, and mononuclear cells. Pericarditis
with fibrinous exudate is common and pathology reveals fibrinoid necrosis
and perivascular infiltration with mononuclear cells. Myocarditis can be seen
as well. SLE poses a very high risk for atherosclerotic coronary artery disease,
and vasculitis, immune complex deposition in addition to corticosteroid use and
hypertension are thought to be contributory.
Lymphadenopathy is common in SLE, and pathology may
reveal follicular hyperplasia with giant cells, plasma cells
infiltration of the interfollicular zones, and necrosis of the
paracortical T-cell zones. LE bodies may be rarely seen. The necrotic vessel
wall shows immunoglobulin and Complement C3 deposition. Splenomegaly is
also common in SLE, with pathology showing the classic onionskin
lesion with has multiple concentric rings of perivascular
collagen. Follicular hyperplasia and periarterial fibrosis are
common.
Lupus pneumonitis can be seen in up to 10% of lupus patients.
Interstitial pneumonitis, alveolitis, alveolar wall injury, and edema and
hemorrhage are commonly seen in these patients. Immunoglobulin and complement
deposition is seen in the vessel wall. Chronic interstitial lung disease can
occur in up to 50% of these patients and is characterized by interstitial
lymphoid aggregates and fibrosis, septal thickening and type-2 pneumocyte
hyperplasia. Medial hypertrophy and intimal fibrosis involving the branches of
the pulmonary artery lead to pulmonary hypertension in SLE. Again,
immunoglobulin and complement deposition can be seen in the vessel wall.
Lupus nephritis can involve the glomeruli, interstitium, tubules
and the vessels with immune complex deposition in all four compartments. The
World Health Organization classification criteria for lupus
nephritis describe 6 classes of lupus nephritis all with distinct
pathological features and significant differences in clinical outcomes. This
has led to a different treatment approach for each class and knowing the class
of lupus nephritis before initiating treatment is vital.
·
Class I: Minimal
mesangial lupus nephritis
·
Class II: Mesangial
proliferative lupus nephritis
·
Class III: Focal
lupus nephritis
·
Class IV: Diffuse
segmental or Diffuse global lupus nephritis
·
Class V: Membranous
lupus nephritis
·
Class VI: Advanced
sclerosing lupus nephritis
Immunohistology
The lupus band test (LBT) Immunoglobulins, predominantly
IgG, but less frequently IgM and IgA, together with complement (C1q, C3) can be
demonstrated at the dermal–epidermal junction by immunofluorescence techniques.
Such deposits are present in lesional skin but are also present in clinically
normal skin of SLE. They occur in more than 80% of skin lesions of DLE and SLE.
If IgG, IgM and IgA are all present, the diagnosis of SLE is likely, and the
more common combination of IgG and IgM is also suggestive. The basement‐membrane
phenomenon can be demonstrated in uninvolved skin in three‐quarters
of active cases of SLE if the biopsy specimens are taken from the exposed skin,
preferably from the dorsum of the wrist or forearm. Biopsy specimens from the
unexposed skin are positive in only approximately 50% of patients. However, a
positive LBT on uninvolved sun‐protected skin is a specific
criterion for identifying patients with LE. In addition to dermal–epidermal
immunoreactant deposition, epidermal nuclear deposits, usually giving a
speckled IgG pattern, occur in the basal epidermal nuclei and cells of the
lower epidermis in nearly one‐third of patients.
Autoantibodies
Non‐organ‐specific humoral auto
antibodies are the hallmark of SLE. A range of auto antibodies may be present
in the disease, although some are more disease‐specific
(anti‐dsDNA
and anti‐Sm
antibodies) and some are much more common (antinuclear and anti‐Ro
antibodies). The B cells that make auto antibodies are activated by elevated
levels of B‐lymphocyte
stimulator (BLys; also known as B‐cell‐activating
factor (BAFF)), a growth factor that is particularly important for the survival
of T‐cell‐dependent
B cells that promote autoantibody formation. Clinical trials have demonstrated
that belimumab, a monoclonal antibody to BAFF, improves mucocutaneous and
arthritis symptoms in patients with SLE. This drug has become the first US Food
and Drug Administration (FDA) approved therapy for SLE.
In SLE, antigen–antibody complexes containing DNA and RNA
products activate the innate immune system through the stimulation of Toll‐like
receptors (TLRs) 9 and 7, respectively. Innate immune activation culminates in
INF‐α
and TNF‐α
release by dendritic cells and this release promotes T cells to release IFN‐γ,
interleukin 6 (IL‐6) and IL‐10;
all cytokines that promote continued antibody formation. The autoantibody‐producing
cells are subsequently inadequately down‐regulated by anti‐idiotypic
antibodies and regulatory T cells.
Clinical features
Due to the clinical and serological diversity, the
disease may affect almost any organ of the body, and can manifest in a broad
variety of ways. Despite the female sex predominance, clinical gender
differences are found in organ involvement and prognosis. Men with lupus tend
to have higher frequencies of renal disease, skin manifestations, cytopenias,
serositis, neurological involvement, thrombosis and vasculitis, with an
increased risk of myocardial infarction, possibly due to an increased frequency
of hypertension and positive lupus anticoagulant.
An autoantibody profile can sometimes be helpful in predicting the
disease course and clinical features. Several studies have indicated the
development of serological abnormalities several years before the onset of
clinical lupus. This is termed as pre-clinical lupus, where a patient may have
serological abnormalities consistent with SLE and may have some clinical
features, but still does not meet the criteria for SLE. There is evidence that
a significant percentage of these patients with pre-clinical lupus that include
those with incomplete lupus, or undifferentiated connective tissue disease may
transition to clinical lupus and fulfill the SLE criteria later in life.
The main clinical features include fever, rashes and
arthritis, but renal, pulmonary, cardiac and neurological involvement may
occur, with increased mortality.
1997 Update of the 1982 American College of Rheumatology revised
criteria for classification of systemic lupus erythematosus.
|
Criterion |
Definition |
|
1. Malar rash |
Fixed erythema, flat or raised, over the malar
eminences, tending the spare the nasolabial folds |
|
2. Discoid rash |
Erythematous raised plaques with adherent keratotic
scaling and follicular plugging; atrophic scarring may occur in older lesions |
|
3.Photosensitivity |
Skin rash as a result of unusual reaction to sunlight,
by patient history or physician observation |
|
4. Oral ulcers |
Oral or nasopharyngeal ulceration, usually painless,
observed by a physician |
|
5. Arthritis |
Non-erosive arthritis involving two or more peripheral
joints, characterized by tenderness, swelling, or effusion |
|
6. Serositis |
a. Pleuritis—convincing history of pleuritic pain or
rub heard by a physician or evidence of pleural effusion |
|
Or |
|
|
b. Pericarditis—documented by electrocardiogram or rub
or evidence of pericardial effusion |
|
|
7. Renal disorder |
a. Persistent proteinuria— >0.5 g/day or greater
than 3+ if quantitation not performed |
|
Or |
|
|
b. Cellular casts—may be red cell, hemoglobin,
granular, tubular, or mixed |
|
|
8. Neurologic disorder |
a. Seizures—in the absence of offending drugs or known
metabolic derangements (e.g., uremia, ketoacidosis, or electrolyte imbalance) |
|
Or |
|
|
b. Psychosis—in the absence of offending drugs or known
metabolic derangements (e.g., uremia, ketoacidosis, or electrolyte imbalance) |
|
|
9. Hematologic disorder |
a. Hemolytic anemia—with reticulocytosis |
|
Or |
|
|
b. Leukopenia— <4,000 μL total on two or more
occasions |
|
|
Or |
|
|
c. Lymphopenia— <1,500/μL on two or more occasions |
|
|
Or |
|
|
d. Thrombocytopenia— <100,000 μL in the absence of
offending drugs |
|
|
10. Immunologic disorder |
a. Anti-DNA—antibody to native DNA in abnormal titer |
|
Or |
|
|
b. Anti-Sm—presence of antibody to Sm nuclear antigen |
|
|
Or |
|
|
c. Positive finding of antiphospholipid antibodies
based on (1) an abnormal serum level of immunoglobulin G or immunoglobulin M
anticardiolipin antibodies, (2) a positive test result for lupus
anticoagulant using a standard method, or (3) a false-positive serologic test
for syphilis known to be positive for at least 6 months and confirmed by
Treponema pallidum immobilization or fluorescent treponemal antibody
absorption test |
|
|
11. Antinuclear antibody |
An abnormal titer of antinuclear antibody by
immunofluorescence of an equivalent assay at any point in time and in the
absence of drugs known to be associated with “drug-induced lupus” syndrome |
* The proposed classification is based on
11 criteria. For the purpose of identifying patients in clinical studies, a
person shall be said to have systemic lupus erythematosus if any four or more
of the 11 criteria are present, serially or simultaneously, during any interval
of observation.
Presentation
The initial manifestations vary. The most commonly
observed presenting symptoms are arthralgia followed by cutaneous involvement.
Presentation with serositis and renal abnormalities are less common.
In fulminating cases there is usually marked
constitutional disturbance, with fever, weight loss, anorexia, malaise and
joint pains; the skin may be involved later, if at all. On the other hand, the
evolution can be gradual, starting with localized skin lesions and systemic
involvement developing later. Fatigue is a prominent symptom, both at
presentation and subsequently. The diagnosis in many cases is made only by
considering the condition in a patient with an obscure illness. As most
patients are female, sex is an important diagnostic point. Although weight loss
is a feature in nearly 50% of cases, some patients may gain weight.
Menstruation is irregular in 18% and absent in 75%.
Clinical manifestations of cumulative systemic lupus
erythematosus and prevalence over the entire course of disease. Adapted from
‘Systemic Lupus Erythematosus’ Harrison's Principles of Internal Medicine, 20th
edition
|
Manifestation |
Prevalence |
||||||||||||||||||
|
1. Systemic: · Fatigue,
malaise, fever, anorexia |
95% |
||||||||||||||||||
|
2. Musculoskeletal: |
95% |
||||||||||||||||||
|
·
Arthralgias/myalgias |
95% |
||||||||||||||||||
|
·
Non‐erosive polyarthritis |
60% |
||||||||||||||||||
|
· Hand
deformities · Myopathy/myositis |
10% 25/5% |
||||||||||||||||||
|
·
Ischaemic necrosis of bone |
15% |
||||||||||||||||||
|
3. Cutaneous: |
80% |
||||||||||||||||||
|
·
Photosensitivity |
70% |
||||||||||||||||||
|
·
Malar rash |
50% |
||||||||||||||||||
|
·
Oral ulcers |
40% |
||||||||||||||||||
|
·
Alopecia |
40% |
||||||||||||||||||
|
·
Discoid/vasculitis rash |
20% |
||||||||||||||||||
|
·
Other |
15% |
||||||||||||||||||
|
4. Haematological: |
85% |
||||||||||||||||||
|
·
Anaemia (chronic disease) |
70% |
||||||||||||||||||
|
·
Leukopenia |
65% |
||||||||||||||||||
|
·
Lymphopenia |
50% |
||||||||||||||||||
|
·
Thrombocytopenia |
15% |
||||||||||||||||||
|
·
Lymphadenopathy |
15% |
||||||||||||||||||
|
·
Splenomegaly |
15% |
||||||||||||||||||
|
·
Haemolyticanaemia |
10% |
||||||||||||||||||
|
5. Neurological: |
60% |
||||||||||||||||||
|
·
Cognitive disorder |
50% |
||||||||||||||||||
|
·
Mood disorder |
40% |
||||||||||||||||||
|
·
Headache |
25% |
||||||||||||||||||
|
·
Seizures |
20% |
||||||||||||||||||
|
·
Mono‐, polyneuropathy |
15% |
||||||||||||||||||
|
·
Stroke, transient ischemic attack |
10% |
||||||||||||||||||
|
·
Acute confused state or movement disorder |
2–5% |
||||||||||||||||||
|
·
Aseptic meningitis, myelopathy |
<1% |
||||||||||||||||||
|
Manifestation |
Prevalence |
||||||||||||||||||
|
6. Cardiopulmonary: |
60% |
||||||||||||||||||
|
· Pleurisy,
pericarditis, effusions |
30–50% |
||||||||||||||||||
|
·
Myocarditis, endocarditis |
10% |
||||||||||||||||||
|
·
Lupus pneumonitis |
10% |
||||||||||||||||||
|
·
Coronary artery disease |
10% |
||||||||||||||||||
|
·
Interstitial fibrosis |
5% |
||||||||||||||||||
|
·
Pulmonary hypertension, acute respiratory
distress syndrome, haemorrhage |
<5% |
||||||||||||||||||
|
·
Shrinking lung syndrome |
<5% |
||||||||||||||||||
|
7. Renal: |
30–50% |
||||||||||||||||||
|
·
Proteinuria ≥500mg/24h, |
30–50% |
||||||||||||||||||
|
·
cellular casts |
|||||||||||||||||||
|
·
Nephrotic syndrome |
25% |
||||||||||||||||||
|
·
Endstage renal disease |
5–10% |
||||||||||||||||||
|
8. Gastrointestinal: |
40% |
||||||||||||||||||
|
· Nonspecific
(nausea, mild pain, diarrhoea)
|
30% |
Constitutional symptoms
Constitutional symptoms are seen in more than 90% of patients with
SLE and are often the initial presenting feature. Fatigue, malaise, fever,
anorexia and weight loss are common. While more than 40% of patients with SLE
may have lupus flare as a cause of fever, infections must always be ruled out
first given the immunocompromised state of these patients. Further, SLE is a
very rare cause of fever of unknown origin.
Mucocutaneous manifestations
Most
of the patients with SLE suffer from mucocutaneous involvement, which is one of
the most well-known and identified clinical features.
Approximately 80% of cases have a rash at some stage, and
in up to 25% it is the presenting sign.
The cutaneous changes described by Gilliam is divided
between: (i) those specific for LE and showing the characteristic
histopathological appearances of interface dermatitis of LE; and (ii) those
that are less specific in their origin and not showing histological changes of
LE and many of these are also seen in the other connective tissue diseases. Non‐specific
LE skin diseases are more frequently associated with SLE than LE‐specific
lesions.
The Gilliam Classification of Skin
Lesions Associated with Lupus Erythematosus
|
LE-Specific
Skin Disease [Cutaneous LE (CLE)] |
LE-Nonspecific
Skin Disease |
|
(A) Acute cutaneous LE (ACLE) 1. Localized
ACLE (malar rash; butterfly rash) 2. Generalized
ACLE (lupus maculopapular lupus rash, SLE rash, rash, photosensitive lupus
dermatitis) (B) Subacute cutaneous LE (SCLE) 1. Annular
SCLE (syn. lupus marginatus, symmetric erythema centrifugum, autoimmune
annular erythema, lupus erythematosus gyrates repens) 2. Papulosquamous
SCLE (syn. disseminated DLE, subacute disseminated LE, superficial
disseminated LE, psoriasiform LE, pityriasiform LE, and maculopapular
photosensitive LE) (C) Chronic cutaneous LE (CCLE) 1. Classic
discoid LE (DLE) · Localized
DLE · Generalized
DLE 2. Hypertrophic/verrucous
DLE 3. Lupus
profundus/lupus panniculitis 4. Mucosal
DLE · Oral
DLE · Conjunctival
DLE 5. Lupus
tumidus (urticarial plaque of LE) 6. Chilblain
LE (chilblain lupus) 7. Lichenoid
DLE (LE/lichen planus overlap, lupus planus) |
(A) Cutaneous vascular disease 1. Vasculitis (a)Leukocytoclastic · Palpable
purpura · Urticarial
vasculitis (b) Periarteritis nodosa-like
cutaneous lesions 2. Vasculopathy (a)Degos disease-like lesions (b)Secondary atrophie blanche (syn. Livedoid
vasculitis, livedo vasculitis) 3. Periungual
telangiectasia 4. Livedo
reticularis 5. Thrombophlebitis 6. Raynaud
phenomenon 7. Erythromelalgia
(erythermalgia) (B) Nonscarring alopecia · “Lupus
hair” · Telogen
effluvium · Alopecia
areata (C) Sclerodactyly (D) Rheumatoid nodules (E) Calcinosis cutis (F) LE-nonspecific bullous lesions (G) Urticaria (H) Papulonodular mucinosis (I) Cutis laxa/anetoderma (J) Acanthosis nigricans (type B insulin resistance) (K) Erythema multiforme (L) Leg ulcers (M) Lichen planus |
Cutaneous features of SLE in 73 patients
|
Cutaneous feature |
Occurrence in SLE (%) |
|
BUTTERFLY RASH AS PART OF ACLE |
51 |
|
Facial edema |
4 |
|
SUBACUTE CUTANEOUS LE |
7 |
|
CHRONIC DLE |
25 |
|
SCARRING DLE ALOPECIA |
14 |
|
Non‐scarring alopecia |
40 |
|
Chilblain lupus |
20 |
|
Mouth ulceration |
31 |
|
Bullous eruptions |
8 |
|
Photosensitivity |
63 |
|
Raynaud's phenomenon |
60 |
|
Chronic urticaria (>36 h) |
44 |
|
Cutaneous vasculitis |
11 |
|
Livedo reticularis |
4 |
|
Episcleritis |
4 |
|
Cheilitis |
4 |
|
The
cutaneous features in bold are considered LE‐specific
skin changes, with the characteristic histology of cutaneous lupus. The other
cutaneous features are considered lupus non‐specific
cutaneous features. |
Lupus‐specific changes
The LE‐specific changes can be
divided into three groups based on the amount of time that the skin symptoms
typically take to present. These include chronic cutaneous LE (CCLE), SCLE and
acute cutaneous LE (ACLE). CCLE includes localized and generalized DLE,
hypertrophic LE, lupus profundus and lupus tumidus. SCLE includes annular and
psoriasiform variants and ACLE includes acute localized and generalized LE, and
toxic epidermal necrolysis (TEN) like variants. Patients with any of these LE‐specific
features may have skin disease alone or SLE if they fulfill the new SLICC
criteria. The risk of SLE with localized versus generalized DLE is 5% versus
20% over time, whereas it is rarely seen with LE tumidus. Lupus panniculitis is
reported to occur in approximately 2–3% of patients with SLE. Similarly,
although the incidence of SLE in patients with SCLE is approximately 50%, only
10–15% has serious organ involvement.
The three major types of cutaneous LE are not mutually
exclusive. In a given patient, more than one type of cutaneous lesion may
occur.
ACLE is often associated with active
SLE. Cutaneous erythema is the most common feature, particularly on light‐exposed areas. Localized
ACLE has commonly been referred to as the classic butterfly rash or malar rash
of SLE (wolf-like erythema is coined). In localized ACLE, confluent symmetric
erythema and edema with mildly scaling are centered over the malar eminences
and bridges over the nose. The nasolabial folds are characteristically spared (photoprotected). In particular, the malar rash is seen
in approximately 60% of patients with SLE, and often a sign of underlying
systemic disease and may fluctuate with lupus
disease activity. It usually has an acute onset and these lesions tend to be transient,
follow sun exposure and lasting only hours, days, or weeks, and resolve without
scarring (but sometimes with dyspigmentation). An association with anti-dsDNA
antibodies and lupus nephritis has been proposed. The presence of
telangiectasias, erosions, dyspigmentation and epidermal atrophy (i.e.
poikiloderma) may help to distinguish the malar erythema of ACLE from that of
common facial eruptions such as seborrheic dermatitis and the vascular type of
rosacea.
In
systemic lupus erythematosus (SLE) (left) the eruption is often just an
erythema, sometimes transient, but occupying most of the ‘butterfly’ area. In
discoid LE (right) the fixed scaling and scarring plaques may occur in the
butterfly area (dotted line), but can occur outside it too.
In generalized ACLE, a widespread brightly erythematous macules
and papules are seen on the photo-exposed areas on the face, upper trunk, and
extensor aspects of the arms and hands and V-shaped region of the neck that can
resemble a viral exanthem or a drug eruption.
Photosensitivity
is present in SLE in more than 90% cases and is characterized by abnormal skin
reaction on exposure to Ultraviolet A/B and visible light, a reaction that may
last weeks to months. These patients also experience worsening of their
systemic symptoms on sun exposure. ACLE is typically
precipitated or exacerbated by exposure to sunlight. UV radiation such as that
found in discos, fluorescent lighting and UVA from photocopiers may also cause
exacerbations. Because UV‐induced lesions of cutaneous LE are
characterized by a latency period of up to several weeks, a negative history of
photosensitivity does not exclude sensitivity to light as the patient may be
unaware of the relationship. Occasionally, more acute lesions with bullae may
follow exposure to the sun, and bullae may be hemorrhagic. Importantly, the
rash in generalized ACLE usually spares the distal interphalangeal, proximal
interphalangeal and metacarpophalangeal joints – an important distinguishing
feature from dermatomyositis.
An extremely acute form of ACLE is rarely seen that can
simulate toxic epidermal necrolysis (TEN). This form of LE-specific
vesiculobullous disease results from widespread apoptosis of epidermal
keratinocytes, and eventuates in areas of full-thickness epidermal skin
necrosis, which is subsequently denuded. This must be differentiated from drug‐induced
TEN in a patient with SLE. Patients with this form of cutaneous LE often occurs
on predominantly sun-exposed skin and has a more insidious onset and have
significant systemic disease activity such as cerebritis or nephritis. In other
cases, lesions are like those of erythema multiforme. The presence of erythema
multiforme-like lesions in lupus patients has been termed Rowell’s syndrome.
Subacute cutaneous lupus erythematosus (SCLE) rash is
photosensitive, widespread, nonscarring, and nonindurated. SCLE may be
either papulosquamous resembling psoriasis or an annular/polycystic lesion with
central clearing and peripheral scaling. SCLE lesions may last several months
but usually, heal without scarring. SCLE rash is seen in patients with a
positive Anti-Ro (SSA) antibody in up to 90% of the cases. SCLE can also
be caused by some drugs such as hydrochlorothiazide. It has also been
reported in patients with Sjogren syndrome and rheumatoid arthritis.
Discoid lupus erythematosus (DLE) is the most common form of
chronic cutaneous lupus erythematosus (CCLE). DLE may occur with or without
SLE, and can be either localized (only head and neck) or generalized (above and
below the neck). The lesions are disk-shaped erythematous papules or plaques
with adherent scaling and central clearing. DLE heals with scarring, and when
present on the scalp, can be associated with permanent alopecia. Mucosal DLE
lesions can be seen in the oral cavity, and these tend to be painful
erythematous round lesions with white radiating hyperkeratotic striae.
Hypertrophic DLE may mimic squamous cell carcinoma histologically. Lupus
panniculitis can occur above the waist and is less likely to be associated with
SLE. The lesions result in depressed areas, and when associated with DLE lesions
overlying them, are known as lupus profundus. Lupus tumidus lesions are
erythematous edematous smooth plaques without epidermal involvement.
Chilblain LE is a rare form of CCLE and presents with
erythematous tender plaques on fingers and toes. Persistence of
lesions beyond the cold months, a positive ANA, or presence of one of the other
criteria for SLE at the time of diagnosis of chilblain lesions helps to
distinguish chilblain LE from idiopathic chilblains. Approximately 20% of
patients presenting with chilblain LE later develop SLE. These lesions may
ulcerate.
Lesions resembling chronic discoid lesions are initial
manifestations in approximately 10% of patients and about 20% of patients with
SLE develop DLE lesions at some point in the course of their disease, and such
patients tend to have less severe forms of SLE.
Lupus non‐specific changes
The presence of
nonspecific LE skin lesions raises the possibility of SLE and may
signify more significant internal disease.
Nonspecific
manifestations of SLE are distinguished by the absence of lupus-specific
changes on histopathology. These include a variety of reactive and inflammatory
eruptions and are typically observed in active SLE.
Reticulate telangiectatic erythema seen on the thenar and
hypothenar eminences of the palms, on the pulps and dorsum of the fingers and,
to a lesser extent, on the toes and over the lateral borders of the feet and
heels and sometimes there may be small vascular necroses on the tips of the
fingers and alongside the nails. Erythromelalgia (erythermalgia) is
characterized by intense burning pain in the feet and hands aggravated by heat
and dependence and relieved by cooling and elevation accompanied by local
macular erythema and warmth may be a presenting feature.
Non‐specific changes in the skin associated
with SLE include nail changes, hair changes, vasculitis, urticarial lesions,
mucinoses, bullous lesions, mucosal lesions and others. These are detailed
below.
Nail changes
Nail changes occur in approximately 25% of patients. Nail
changes in SLE includes nail fold erythema, red lunulae, nail fold hyperkeratosis
and ragged cuticles. Other nail findings include nail ridging, onycholysis,
onychomadesis and punctate or striate leukonychia caused by altered
keratinization of the nail matrix. Blue‐black nail
pigmentation may also be observed, and is thought to occur from increased
melanin deposition. This dyschromia may be diffuse or longitudinal and may be
caused by medications, most frequently antimalarials, but occasionally
associated with methotrexate, cyclophosphamide and gold. Capillaroscopy may
reveal glomerulization of the capillaries. Telangiectasia and erythema of the
proximal nail fold are found in 76% of patients who had both DLE and SLE, but
none in patients with DLE in the absence of SLE, suggesting that it, too, is a
rather sensitive indicator for systemic disease activity.
Hair changes
These changes can be scarring or non‐scarring.
The most common non‐specific skin manifestation of SLE is the
diffuse, non‐scarring
loss of hair with a reddish scalp known as telogen effluvium which occurs in
more than 60% of patients, especially in the active phase of the disease or,
less frequently, permanent scarring alopecia is found in DLE lesions on the
scalp. Alternatively, the alopecia can be chronic leading to coarse, dry and
fragile hair, especially on the frontal margin. This leads to an unruly
appearance with short, broken-off hair, the so-called ‘lupus hair’. This occurs
in 30% of patients, predominantly females. The hair loss recovers as the
disease becomes inactive, but ‘lupus hair’ usually persists longer than
alopecia. Alopecia areata is also found in approximately 10% of patients with
SLE.
Cutaneous vascular reactions
They can be divided into vasculitis or vasculopathy and
the distinction between the two conditions is important as their management is
distinctly different. Vasculitis is caused by primary inflammation of the vessel
walls with secondary occlusion by fibrin, whereas vasculopathy can be defined
as narrowing of the vessel walls (i.e. ischaemic) or non‐inflammatory
vessel lumen occlusion from thromboembolic disease.
Vasculitis
Arterioles and venules of the skin are frequently
affected in SLE; all sizes of blood vessels may be affected. Vasculitis in SLE
usually presents as a small‐vessel leukocytoclastic vasculitis
with palpable purpura in dependent areas which is seen in 8–11% of patients
with SLE. Cutaneous vasculitis in a patient with SLE may predict the
development of lupus nephritis. Chronic urticaria occurs in 44% of SLE patients
and is considered to be brought about by immune-complex deposition. Clinically,
lesions may be indistinguishable from typical hives; but unlike hives, they are
usually nonpruritic, painful and tender urticarial lesions, often over bony
prominences, which last longer than 24 hours. It often involves nondependent
areas of skin that characteristically leaves post‐inflammatory
hyper pigmentation or purpura. This clinical presentation is typical of
urticarial vasculitis. In most cases a biopsy reveals necrotizing vasculitis,
and the lupus band test is generally positive. Involvement of medium and/or
large vessels in the skin, may present as purpuric plaques with stellate or
retiform borders with or without necrosis and ulceration, or as subcutaneous
nodules.
Vasculopathy
Raynaud phenomenon can be seen in 25–60% of patients with
SLE and is the most common nonspecific findings in such patients. It is
characterized by reversible vasospasm of the fingers and toes, often caused by
cold exposure, with triphasic color change; there is cold‐induced
pallor, followed by cyanosis pain and numbness, then erythematous discoloration
on rewarming. Predictors associated with SLE and Raynaud phenomenon on include
persistent periungual telangiectasia, involvement of the thumbs, ears, nose and
toes, ice‐pick
or pitted scarring of the pulps and high ANAs, anti‐RNP
and nucleolar antibodies. Raynaud's phenomenon seems to herald a worse
prognosis and is associated with higher disease activity scores. Raynaud
phenomenon on in SLE is also associated with migraine and pulmonary artery
hypertension.
Livedo reticularis is seen in up to 35% of SLE patients.
It may be seen in patients with SLE both with and without the antiphospholipid
syndrome. It presents as a fishnet‐like, complete or
incomplete, red-purple rings, which blanches on pressure, and is not affected
by temperature changes, may develop, most commonly on the buttocks and legs,
followed by the outer aspects of the arms and less commonly on the trunk.
Superficial ulceration can occur in areas of livedo. The net‐like
discoloration results from hypo‐oxygenation due to slow
arterial blood flow in the dermal arterioles and the increased collection of
the hypo‐oxygenated
blood in the dermal venules. Livedo racemosa is distinguished from livedo reticularis
based on a ‘broken net’ type of pattern and is thought to be a sign of more
severe disease due to the presence of cholesterol and fibrin thrombi and
calcification in the vessels. The appearance of livedo reticularis in patients
with SLE and antiphospholipid syndrome may herald central nervous system
involvement. Catastrophic antiphospholipid syndrome (CAPS) is rare (<1%),
but has a high mortality of approximately 50%. CAPS presents in patients with
APAb syndrome with a disseminated, intravascular, coagulation‐type
picture with purpura fulminans. The diagnosis is made based on evidence of
thrombosis in at least three organs, a histological finding of small‐vessel
occlusion in at least one organ, a laboratory confirmation of APAbs and the
rapid development of clinical manifestations
Atrophie blanche‐type lesions
(painful, ivory, stellate scars on the lower extremities) may occur. Lesions
similar to those in Degos disease (malignant atrophic papulosis;) – small,
porcelain‐white,
atrophic macules with peripheral erythema and telangiectasia – may also occur
in patients with APAbs. In SLE patients with Degos‐like
lesions, a more benign course is usual without the characteristic visceral
involvement (digestive tract or central nervous system) that is commonly
described in Degos syndrome. Lesions of primary anetoderma may also be seen and
consist of localized areas of herniated sac‐like or flaccid skin
as a result of localized elastic loss.
Large vessel disease
Gangrene of the tips of the fingers and toes may develop
insidiously. At first the digits become blue and cold and may be painful.
Radiography of the fingers in cases with peripheral ischemia shows absorption of
the distal part of the terminal phalanges, as in systemic sclerosis. Later, the
phalanges may become exposed, and spontaneous separation of the tips of the
fingers may occur. Amputation of digits may be required. Occlusion of large- and
medium-sized arteries can occur suddenly and result in gangrene requiring
amputation of a limb. This may be the result of vasculitis or thrombosis.
Patients with thrombosis frequently have antiphospholipid antibodies. Leg
ulcers occur in approximately 10% of patients, usually near the malleoli but
sometimes on the feet and elsewhere, from breakdown in reticular livedo and in
areas of cutaneous vasculitis.
Mucinosis
Although mucin deposits are a common and often prominent
histological feature of cutaneous lupus, specific clinical patterns of
mucinosis also occur. Papular or nodular lesions resulting from mucinous
deposits in the dermis (papulonodular mucinosis) without microscopic features
of LE have been reported and form a distinct entity, which may be the
presenting feature of LE. It presents as multiple, asymptomatic, firm flesh‐colored
dermal papules and nodules, usually on the trunk, arms or head and neck, and
can be associated with SLE, SCLE or DLE or can occur alone. The overlying
epidermis appeared normal.
Other connective tissue changes
Dystrophic calcinosis is rare, and is most often seen in
association with lupus panniculitis but can also occur in association with SLE.
It occurs most often on the extremities and buttocks as asymptomatic nodules
discovered by radiology. Occasionally, the skin overlying the calcinosis can
ulcerate, leading to the extrusion of chalk‐like material. The
mechanism underlying calcinosis cutis in SLE is unknown, but may be due to
increased calcium concentration in the presence of necrotic and apoptotic
cells, secondary to trauma or tissue damage. Subcutaneous nodules occur in
approximately 5% of patients. They occur mainly over the backs of the proximal
phalangeal joints and wrists, but are also found on the elbows, knees, occiput
and flexor aspects of the fingers. They may resemble rheumatoid nodules and can
respond to hydroxychloroquine. Some are identical histologically with classic
rheumatoid nodules, while others are probably caused by vasculitis and
thrombosis.
Bullous lesions
Blistering is uncommon in SLE and can be divided into
three categories:
1. Subepidermal
bullae in ACLE and SCLE lesions due to separation of the epidermis and dermis
as a result of severe liquefaction degeneration of the basal layer and dermal edema
(TEN‐like
ACLE and Rowell syndrome).
2. SLE‐associated
autoimmune bullous disease including dermatitis herpetiformis, pemphigus
vulgaris (so‐called
pemphigus erythematosus), pemphigus foliaceus, paraneoplastic pemphigus,
bullous pemphigoid, pseudoporphria, epidermolysis bullosa acquisita and IgA
disease.
3. A
separate subset, bullous SLE (BSLE), is a distinct type of non‐specific,
autoantibody‐mediated,
cutaneous SLE that results in a subepidermal blister.
The diagnosis of BSLE requires the
presence of: (i) SLE; (ii) a vesiculobullous eruption arising but not limited
to sun‐exposed skin; (iii)
histopathological subepidermal bulla containing neutrophils. Neutrophils
accumulate within dermal papillae forming neutrophilic microabscesses, often
resembles dermatitis herpetiformis; and (iv) DIF demonstrates linear IgG (± IgA
and IgM or C3) deposits at the basement‐membrane zone, contrasting with the granular
immunostaining seen in LE interface dermatitis. Electron microscopy shows the
immunoreactants to be in the sublamina densa. The disease is mediated by
circulating antibodies against type VII collagen as demonstrated by indirect immunofluorescence
(IDIF).
Clinically, the bullous lesions arise predominantly on
normal or erythematous sun‐exposed skin on the face,
neck and upper trunk or flexural skin, but they may be more widespread, and can
heal with milia formation. Glomerulonephritis is common. Given that BSLE occurs
in the setting of SLE, the ANA test is generally positive, and anti‐dsDNA,
anti‐Sm,
anti‐Ro,
anti‐La
and anticardiolipin antibodies may also be detected. Low complement,
proteinuria or cellular casts on urinalysis as well as hematological
abnormalities may also reflect disease activity.
Dapsone either alone or in combination with prednisolone
is the treatment of choice. The response may be dramatic, with cessation of new
bullae within 1–2 days. However rapid recurrence may occur upon withdrawal of
dapsone, with remission after re‐initiation of
therapy.
Pemphigus erythematosus combines the immunological
features of pemphigus and LE and presents with erythematous, scaly,
hyperkeratotic or crusted lesions, sometimes adversely affected by the sun,
occur in a butterfly distribution on the cheeks and in a seborrhoeic
distribution on the trunk of patients with Senear–Usher syndrome. DIF
demonstrates immunoglobulin and complement in the intercellular substance and
at the dermal–epidermal junction of perilesional and, to a lesser extent, of
light‐exposed
and non‐exposed
skin. Circulating pemphigus‐like antibodies and
antinuclear factor occur in 80–100% of patients, but anti‐DNA
and ENA antibodies are not found. Antidesmoglein antibodies 1 and 3 have also
been demonstrated. The condition occurs spontaneously, but has been induced by
penicillamine, propranolol, captopril, pyritinolol and thiopronine. Topical
steroids alone may control the condition, but systemic steroids,
immunosuppressives or dapsone may be required.
Oral and naso‐pharyngeal ulcers are
one of the SLICC criteria for identifying patients with SLE and are found in
active phase of the disease. Lesions start as small erythematous or purpuric
areas, which break down to form shallow ulcers, with a dirty yellow base and
surrounding reddish halo. LE ulcers are usually painless and can affect any part of the oral cavity with
most common locations being the hard palate, the buccal mucosa, and the
vermilion border. Histology
and immunohistology show changes similar to those in the skin. DIF is usually
positive. Repeated sore throats and oral ulceration may be presenting features.
Chelitis occurs in approximately 4%, the lips having a silvery appearance, with
erythema, scaling and blurring of the vermilion border. The larynx is
occasionally involved. Ulceration of the mucosa of the nasal septum occurs in
approximately 5%. Perforation of the nasal septum is a complication of
exacerbations and presents with epistaxis. Erythema of the vulva and perianal
area occurs and vulval ulceration may develop, but is less common than oral
ulcers.
Given the potential risk of transformation to squamous
cell carcinoma in these patients (increased age >60 years), mucosal biopsy
should be considered in any non‐healing ulcerated lesion.
Musculoskeletal manifestations
Approximately 80% to 90% of patients with SLE suffer from
musculoskeletal involvement at some point during their disease course and may
range from mild arthralgias to deforming arthritis. Lupus arthritis is
typically a non-erosive, symmetrical inflammatory polyarthritis affecting
predominantly the small joints of the hands, knees, and wrists, although any
joint can be involved. Jaccoud arthropathy is the result of the joint
capsule and ligament laxity leading to non-erosive deformities of the hands
including ulnar deviation and subluxation of the metacarpophalangeal joints
that may mimic rheumatoid arthritis. Usually, these deformities are reducible,
although rarely, they may become fixed. Avascular necrosis (with or without
steroid use) can occur in up to 10% of patients with SLE and is usually
bilateral and involves the hip joints. Inflammatory myopathy with
histopathological features similar to but less striking than polymyositis has
been seen in less than 10% of SLE cases. Patients with SLE are at high risk for
the development of fibromyalgia with incidences as high as 20% reported.
Rheumatoid nodules have been reported in patients with SLE.
Hematologic manifestations
Anemia is present in more than 50 % of patients with SLE and most
commonly is anemia of chronic disease. Other causes of anemia in SLE may
include iron deficiency anemia, coomb's positive autoimmune hemolytic anemia,
red blood cell aplasia and microangiopathic hemolytic anemia which may be
associated with antiphospholipid antibody syndrome. Leukopenia secondary
to neutropenia or lymphopenia is also very frequent and can be severe.
Thrombocytopenia can be mild or severe and may be associated
with antiphospholipid antibody syndrome and autoantibodies against
platelets, glycoprotein IIb/IIIa or thrombopoietin receptor.
Pancytopenia is not infrequent and may occasionally be associated with
myelofibrosis. Soft non-tender lymphadenopathy is common in SLE, although rare
cases of histiocytic necrotizing lymphadenitis have been reported (Kikuchi-Fujimoto
disease). Splenomegaly is common in SLE, while splenic atrophy and asplenism
have been reported.
Neuropsychiatric manifestations
Both central (CNS) and peripheral (PNS) nervous systems may be
involved in SLE in addition to several psychiatric manifestations, although the
diagnosis can be difficult. The most common CNS manifestation is intractable
headaches, reported in more than 50% cases. Focal or generalized seizures
may be seen, and are associated with disease activity, although they carry a
favorable prognosis. Other CNS manifestations include aseptic meningitis,
demyelinating syndrome including optic neuritis and myelitis, movement
disorders such as chorea and cognitive dysfunction. Patients with SLE are also
at high risk for ischemic strokes. Cranial and peripheral (sensorimotor,
axonal) neuropathies, mononeuritis multiplex, autonomic neuropathies, and
syndromes mimicking Guillain-Barré syndrome and Myasthenia gravis are
the peripheral nervous system manifestations. Psychiatric manifestations are
especially difficult to diagnose and manage and may range from depression and
anxiety to frank psychosis.
Renal manifestations
Lupus nephritis is a well-known and common complication of SLE.
The involvement may range from mild subnephrotic proteinuria to diffuse
progressive glomerulonephritis leading to chronic kidney damage. Lupus
nephritis usually occurs early in the course of SLE. New-onset hypertension,
hematuria, proteinuria, lower extremity edema, and elevation in creatinine
shall raise suspicion for lupus nephritis. A biopsy is crucial in staging the
lupus nephritis and ruling out other causes. The six classes of lupus nephritis
are mentioned in the histopathology section of this article. The treatment of
lupus nephritis is dictated by the biopsy findings and prognosis varies for
each class with an excellent prognosis for classes I and II, and poor outcomes
with classes III and IV. Class V usually carries a favorable prognosis except
for complications of nephritis syndrome such as thromboembolism which are
common in this class. Other renal manifestations may include thrombotic
microangiopathy, interstitial nephritis, lupus vasculopathy, vasculitis,
and arteriolosclerosis.
Pulmonary manifestations
Pleuritis is the most common pulmonary manifestation, and may not
always be associated with pleural effusion. Other pulmonary manifestations
include exudative pleural effusions, acute lupus pneumonitis with bilateral
pulmonary infiltrates, interstitial lung disease which may be nonspecific
interstitial pneumonia (NSIP) or usual interstitial pneumonia (UIP), diffuse
alveolar hemorrhage associated with capillaritis, pulmonary arterial
hypertension, pulmonary embolism (with or without antiphospholipid antibody
syndrome) and shrinking lung syndrome.
Cardiovascular manifestations
SLE may involve any layer of the heart including the pericardium,
myocardium, endocardium and even the coronary arteries. Pericarditis associated
with exudative pericardial effusions is the most common cardiac manifestation.
Cardiac tamponade is rare. Myocarditis is rare and is associated
with anti-Ro (SSA) antibodies. Hydroxychloroquine associated
cardiomyopathy shall be ruled out and this may occasionally require an
endomyocardial biopsy. Valvular abnormalities including Libman-Sacks endocarditis
involving the mitral valve are common and may be associated
with antiphospholipid antibody syndrome. Patients with SLE are especially
at high risk for coronary artery disease, either due to coronary vasculitis or
more frequently due to generalized atherosclerosis.
Gastrointestinal manifestations
Any part of the gastrointestinal tract may be involved in SLE and
these manifestations include esophageal dysmotility (especially the
upper one-third part of the esophagus), mesenteric vasculitis, lupus
enteritis, peritonitis and ascites, protein-losing enteropathy, pancreatitis,
and lupoid hepatitis. Further, patients with SLE and antiphospholipid antibody
syndrome can develop Budd-Chiari syndrome, mesenteric vessel thrombosis, and
hepatic veno-occlusive disease.
Other manifestations
Eye involvement is common and keratoconjunctivitis sicca is
frequently seen in SLE, in presence or absence of secondary Sjogren syndrome.
Retinal vasculitis, optic neuritis, uveitis, scleritis, peripheral ulcerative
keratitis, and episcleritis are other ocular manifestations. Patients with SLE
are also more susceptible to drug-induced ocular damage including
steroid-induced glaucoma or cataract and hydroxychloroquine induced
maculopathy. Ear involvement may lead to sudden sensorineural hearing loss.
Adrenal infarction secondary to adrenal vessel thrombosis may be seen in
patients with SLE and antiphospholipid antibody syndrome.
Differential Diagnosis
SLE
is a systemic disease with multi organ involvement, and several other diseases
can mimic SLE. The manifestations of SLE are so varied and
protean that the disease is often referred to as ‘the great mimicker’.
·
Other
autoimmune diseases
o Rheumatoid arthritis (RA) can present with
several extra-articular manifestations in addition to the classic polyarticular
inflammatory arthritis and may be difficult to differentiate from SLE. Positive
ANA, Anti-Ro, and Anti-La can also be seen in RA although other SLE specific
auto antibodies and hypocomplementemia are rare. SLE can be associated with a
positive rheumatoid factor, but the Anti-CCP is negative in SLE
o Drug-induced lupus may be difficult to
differentiate from SLE especially due to a significant overlap in the clinical
and serological features. Drug-induced lupus is characterized by the resolution
of symptoms after drug withdrawal and lack of more severe manifestations
although the auto antibodies may remain positive for several years.
o Adult-onset Still disease characterized by
arthralgia, fever, lymphadenopathy, and splenomegaly but no malar rash or other
organ manifestations and lacks the SLE specific auto antibodies.
o Behcet disease presents with aphthous ulcers,
uveitis, and arthralgia, but lacks the other systemic and serological features
of SLE.
o Sarcoidosis presents with fever, cough, dyspnea,
fatigue, night sweats, rash, and uveitis. It shows non-caseating granuloma on
chest radiography and bilateral adenopathy, which is rarely present in SLE.
·
Infections
o Several viral infections can mimic SLE.
Parvovirus B19 infection can cause fever, rash, inflammatory arthritis and
cytopenias. ANA and rheumatoid factor have been reported. Hepatitis B and C can
be associated with arthralgia/inflammatory arthritis and positive ANA and
rheumatoid factor. CMV and EBV viral infections can cause fever, fatigue,
cytopenias, and transaminitis. HIV can cause fever, fatigue, oral ulcers, and
cytopenias. More specific autoantibodies and systemic manifestations of SLE are
absent in these viral infections. Further, positive viral serologies may help
make the right diagnosis.
o Infectious endocarditis characterized by fever,
arterial emboli, arthralgia, myalgia, and a heart murmur; may be confused
with cardiac manifestations of SLE, but can be differentiated by the
absence of specific SLE associated auto antibodies and positive blood cultures.
·
Malignancies
o Lymphomas especially Non-Hodgkins lymphoma can
present with fatigue, weight loss, fever, arthralgia, cytopenia,
lymphadenopathy, and a positive ANA. The more specific SLE associated auto antibodies
are absent. In elderly patients presenting with lupus-like symptoms, malignancy
shall be rule out by cancer screening.
|
CLASSIC DISTINCTIONS BETWEEN
DRUG-INDUCED SUBACUTE CUTANEOUS LUPUS ERYTHEMATOSUS (DI-SCLE) AND
DRUG-INDUCED SYSTEMIC LUPUS ERYTHEMATOSUS (DI-SLE) |
||||
|
Disease |
Most commonly associated medications |
Cutaneous findings |
Systemic findings |
Associated autoantibodies |
|
DI-SCLE |
Terbinafine Thiazide diuretics
(e.g. hydrochlorothiazide) TNF-α inhibitors Proton pump
inhibitors (e.g. lansoprazole, pantoprazole, omeprazole) Calcium channel
blockers (e.g. diltiazem, nifedipine, verapamil) Anti-epileptics
(e.g. carbamazepine) |
SCLE |
Usually none |
Anti-SSA/Ro |
|
DI-SLE |
Hydralazine, procainamide, isoniazid, quinidine,
methyldopa, chlorpromazine, minocycline, TNF-α inhibitors* |
Usually absent |
Serositis (arthralgia, pleuritis, pericarditis) |
Anti-histone** |
* TNF-α inhibitors have been associated with DI-SCLE and
DI-SLE (as well as drug-induced discoid lupus erythematosus).
** Anti-histone antibodies are not specific for DI-SLE as
they are commonly found in patients with classic SLE, as well as in other
autoimmune connective tissue diseases.
Complications and co‐morbidities
Complications in patients with SLE may occur either from a result
of organ damage by the disease or due to the adverse effects of the
medications.
Disease process-related complications include but are not limited
to accelerated atherosclerosis with a several-fold higher risk of coronary
artery disease even in the younger population, end-stage renal disease, and
neurological deficits including blindness secondary to neuropsychiatric
manifestations. Patients with severe cutaneous lupus especially discoid lupus
can suffer from permanent skin damage and alopecia. Anxiety and depression are
more common in patients with SLE. Several pregnancy-related complications
are well known including fetal loss, pre-eclampsia and eclampsia, congenital
heart block and neonatal lupus.
Medication-induced complications are common and require close
monitoring. Long-term corticosteroid use in SLE patients frequently leads to
osteoporosis which is under-diagnosed and under-treated leading to osteoporotic
fractures. Other complications of long-term use corticosteroid therapy include
avascular necrosis, glaucoma, cataract, weight gain and poor control of
Diabetes mellitus. High dose corticosteroid use can also be associated with
opportunistic infections and acute psychosis. Long term use of
hydroxychloroquine may rarely result in maculopathy and retinopathy that is
irreversible, and close ophthalmology examinations are recommended.
Cyclophosphamide use is associated with a significantly high risk of
interstitial cystitis and bladder cancer even after drug discontinuation. SLE
patients are immunocompromised and at a significantly high risk of infections
which is one of the major causes of morbidity and mortality in SLE.
SLE in childhood
Approximately 15–20% of SLE has its onset in childhood.
The clinical picture, course and treatment are similar to the disorder in
adults, but on the whole children have more severe disease. Malar rash,
mucocutaneous involvement, hematological abnormalities, seizures, renal involvement
and fever are more common in children. Enlargement of the liver, spleen and
lymph nodes are also more common in childhood cases. The prognosis of patients
with renal disease is now better than earlier reports suggested. Prolonged therapy with high‐dose
steroids may increase disease‐related damage; this may be
avoided by judicious use of immunosuppressives.
SLE in the elderly
Women of child-bearing age
are most often affected; however, approximately 10-20% of cases occur in older
patients. Elderly-onset lupus has been defined in various studies as onset of
lupus after age 50-65 years. Menopause and changes in cellular immunity with
aging may contribute to development of lupus in older adults. Many studies suggest
that the clinical and serological features of elderly-onset lupus differ from
those of lupus in younger patients. Arthritis, fever, serositis, sicca
symptoms, Raynaud's syndrome, lung disease and neuropsychiatric symptoms are
more common in patients with elderly-onset lupus, while malar rash, discoid
lupus and glomerulonephritis are less common in elderly-onset patients compared
with younger lupus patients. Most elderly-onset lupus patients have a positive
anti-nuclear antibody test, but the prevalence of anti-double-stranded DNA and
hypocomplementaemia is lower in elderly-onset patients than in younger
patients. Rheumatoid factor, anti-Ro/Sjögren's syndrome (SS) A and anti-La/SSB
are more often positive in elderly-onset patients. The diagnosis of elderly-onset
lupus may be delayed for many months: insidious onset, low prevalence and
similarity to other more common disorders make the diagnosis of lupus
challenging in this population. Treatment of lupus in the elderly may be
complicated by co-morbidities and increased risk of toxicities from usual
treatments. Optimal management of elderly-onset lupus is empiric because of a
lack of randomized controlled studies. However, the approach to treatment is
similar regardless of the age of the patient.
SLE in pregnancy
Fertility is normal if renal function
is good. Worsening of SLE is uncommon in pregnancy, especially in those on
immunosuppressive therapy. Clinical remission for 6 months before conception
should indicate an uncomplicated pregnancy and a live birth. Anti-Ro (SSA) and Anti-La (SSB) antibodies can
cross the placenta leading to fetal heart block and neonatal lupus
presenting with a photosensitive rash, cytopenias, and transaminitis. The risk
is 2% with the first pregnancy but increases to 20% if a history of neonatal
lupus in past pregnancy. There
is a higher risk of complicated pregnancies in SLE in all patients, regardless
of whether or not SLE is active. Active lupus nephritis poses the greatest risk
to pregnancy outcomes in lupus. Lupus nephritis can be difficult to differentiate from
pre-eclampsia although several clinical and laboratory features (low
complements, positive Anti-Ds-DNA antibody, normal serum uric acid level, and
active urinary sediment) may help. There is a higher risk of premature delivery,
fetal loss and perinatal mortality in all patients. The increased risk of fetal
death may be because of immune complex deposition on the trophoblast basement
membrane, or the transplacental passage of antiphospholipid antibodies. SLE patients with positive antiphospholipid
antibodies are at a high risk of spontaneous abortions and fetal loss,
pre-eclampsia and maternal thrombosis, but the presence of these antibodies
without a previous history of fetal loss or vascular thrombosis is not an
indication for treatment. With a history of recurrent fetal loss or vascular
thrombosis, treatment with low‐molecular‐weight heparin (LMWH)
and low‐dose aspirin may be
effective. Patients with more
severe SLE manifestations such as pulmonary hypertension, severe cardiovascular
disease or cerebrovascular accident are especially at a very high risk of
mortality during pregnancy.
Disease course and
prognosis
Despite the advancements in therapeutic options of SLE and a
better understanding of the disease process, SLE patients suffer from
significant morbidity and carry a high mortality. Early diagnosis with therapy aimed at
preventing organ damage, monitoring and screening patients for cardiovascular
disease and infections with early intervention may improve these outcomes.
The course of SLE is very variable. Acute fulminating
cases are much less common than subacute cases, which smoulder on for many
years. Approximately three-quarters will now survive 15 years. Survival is
related to organ involvement and to the frequency of exacerbations. Of those
without renal involvement, 84% survive 15 years, compared with 57% whose
kidneys are affected. The highest standard mortality rates (SMRs) are seen in
female patients, those of younger age, those with SLE duration <1 year. Serological
as well as clinical remission is uncommon. Exacerbations are more frequent in
the first 5 years of the disease. Pregnancy does not affect long‐term
survival. Prolonged survival is associated with an increased risk of
atherosclerosis, avascular necrosis and neuropsychiatric dysfunction. In
elderly people the presentation is insidious and the clinical course is
relatively benign. Renal disease and serological abnormalities are less
frequent, and arthritis, with subcutaneous nodules, and pleuropericarditis are
more prominent in elderly people.
The better prognosis of the more recent series is a
result not only of the administration of corticosteroids, but also of earlier
diagnosis, the avoidance of stress and drugs such as sulphonamides, and the
control of infections by antibiotics. Persistent causes of death include renal
disease, severe lupus disease activity, infection and cardiovascular disease.
In an international study, a lower total cancer mortality risk in SLE was
observed with an increased mortality from hematological cancers such as non‐Hodgkin
lymphoma and lung cancer but a decreased mortality from breast cancer.
The
spectrum of lupus erythematosus, as envisaged by the late Dr. James N. Gilliam
The
left comprises conditions that define cutaneous disease only and it can be seen
that chronic cutaneous lupus extends into the systemic disease section. This is
also true for Iupus profundus (lupus panniculitis) and subacute cutaneous
lupus, whereas acute cutaneous lupus is characteristic for systemic disease
only. The bottom shows that immune complex disease dominates systemic disease
and cell-mediated immunity (CMI) is predominant in the cutaneous disease
manifestations.
Evaluation
The diagnosis of SLE can be challenging, and no single clinical
feature or lab abnormality can confirm a diagnosis of SLE. SLE is diagnosed
based on the constellation of signs, symptoms and appropriate laboratory
workup. Imaging and histopathology may play a crucial role as well.
Connective tissue
laboratory screening tests
The signs and symptoms often associated with
connective tissue disease (fatigue, arthralgias, fever, and weight loss) are
not specific for autoimmune disease and occur in many other diseases. This
makes early and accurate diagnosis difficult. The Mayo Medical Laboratories
developed a Connective Tissue Diseases Cascade of tests for the primary care
physician to evaluate patients with signs and symptoms compatible with a
connective tissue disease in a setting of low disease prevalence. It provides
immediate disease-specific, follow-up tests in those patients with presumptive
serologic evidence of disease.
Antinuclear antibody
screening
Several auto antibodies have been described in SLE, with varying
degrees of sensitivity and specificity. While some auto antibodies may be
associated with a certain clinical subset of SLE, others may serve as a marker
of disease activity.
Following clinical assessment, if the pretest probability
of SLE is high, ANA testing is ordered to support the diagnosis.
ANA is the first test to order when
autoimmune connective tissue disease is suspected. ANA tests identify
antibodies present in serum that bind to auto antigens present in the nuclei of
mammalian cells.
Immunofluorescence assay is
considered the gold standard test for ANA, and although other methods of detection
such as ELISAs and multiplex assays are widely available, they lack
sensitivity. A positive ANA is seen in more than 97% of cases of SLE, although
it can also be seen in several other disorders, as well as a significant
proportion of the healthy population and have a specificity of only 20%. Hence,
a positive ANA does not confirm the diagnosis of SLE, but a negative ANA makes
it very less likely. ANA negative SLE has been rarely described, although it is
considered to be mostly due to methodical error and those cases have either a
positive ANA on immunofluorescence, or have a positive Anti-Ro (SSA) antibody.
A negative result suggests that an autoimmune
connective tissue disease is unlikely; a positive result, especially a high
titer in a patient with appropriate clinical findings, supports a diagnosis of
an autoimmune connective tissue disease.
False-positive test results
Positive results occur in normal blood donors
and in patients with chronic liver disease, neoplasms, or active chronic
infections. These patients usually have lower titers than those in patients
with autoimmune diseases. Any autoantibody test must be interpreted in the
context of available clinical information.
Specific diagnostic antibody tests
Specific
antibody tests should be ordered. The clinical presentation and ANA pattern
help to determine the tests that should be ordered.
Titers
Specimens are screened by diluting them 1:40
with saline. If there is no nuclear fluorescence at this dilution, the result
is reported as “negative.” If there is green fluorescence, the level of ANA
present is determined by repeating the test after serially diluting the
specimen. ANA results are now reported by many laboratories using the
international unit system (e.g., 1 international unit rather than a titer of
160).
Patterns
Antibodies to nuclear
antigens attach to the various components of the nucleus. The
fluorescein-labeled antihuman immunoglobulins are applied to the preparation
and react with ANAs that have attached to the nucleus. The preparation is
visualized with a fluorescent microscope. Diverse patterns of nuclear
fluorescence (homogeneous, peripheral, speckled, or nucleolar) reflect the
binding of antibodies to different nuclear components. Nuclear staining
patterns were once used as criteria for sub setting, but, with the availability
of direct measurements for specific auto antibodies, pattern identification has
become less important. The test requires interpretation by visual inspection
and consequently lacks a high degree of specificity.
One or more ANAs can be detected by fluorescent antibody
techniques in over 80% of cases. The incidence depends on the substrate used.
Most laboratories now use human cell lines for antibody testing, particularly
Hep‐2
cells derived from a human laryngeal cell line. This produces a reduction in
the proportion of patients said to be antinuclear factor negative. Five
staining patterns of ANAs are demonstrated:
Immunofluorescence Patterns of ANA
in SLE and Other Connective Tissue Diseases
Several
patterns of ANAs have been reported including speckled, homogenous, centromere,
nucleolar, and peripheral patterns. These staining patterns are
produced by separate antibodies, but more than one antibody may be present in a
single serum, usually in different titers. No particular antibody is specific
for any disease.
With the availability of more specific ANAs targeting
specific antigens, the staining patterns of ANAs are not considered significant
enough by themselves. The speckled pattern is seen when ANAs are directed
against the antigens such as SSA, SSB, Smith, Ribonucleoprotein. The homogenous
pattern is associated with ANAs targeted at Histones, Chromatin, and Ds-DNA,
while centromere pattern is associated with Anti-centromere antibodies seen in
limited systemic sclerosis.
Homogeneous antinuclear factor (which is the same factor
as the LE cell factor) is more than twice as common as the speckled factor, but
antinucleolar antibody is only occasionally found. The peripheral factor is
present in high titer in more than 50% of cases in the active phase of the
disease and is infrequent in other diseases. The so‐called
shrunken peripheral pattern is thought to be associated with a poor prognosis
and a high incidence of renal disease. It may appear 10–15 days before an
exacerbation of the disease and be associated with a fall in serum complement.
A high titer (over 1: 64) of antinuclear factor(s) in a patient with the
symptoms and signs of a multiple system disorder suggests the possibility of
SLE or systemic sclerosis, and almost certainly excludes polyarteritis nodosa
or primary cutaneous vasculitis. Any person in apparently good health found to
have a high titer of antinuclear factor should be followed up for years, as
there is a considerable likelihood of developing LE or systemic sclerosis. On
the other hand, a low titer (less than 1: 16), in the absence of clinical
symptoms and signs, can be ignored.
A positive ANA shall be followed by testing for more specific auto
antibodies to detect the antigen responsible for the positive ANA. It must be
noted that frequently, a positive ANA will not be associated with any of the
known more specific auto antibodies. There are several possible targets for
ANAs, with any peptide synthesized inside the nucleus of the cell serving as a
potential antigen, however, so far, only a few have been identified as having
clinical relevance. A positive ANA with negative testing for more specific
autoantibody testing is less likely to be associated with systemic autoimmune
disease.
Circulating Anti-Ds-DNA antibodies are almost always
present in active disease and have
more than 95% specificity for SLE but are seen in only about 60% to 70% of SLE
patients. Anti-Ds-DNA antibodies may occur in the absence of antinuclear
factors, although this is very uncommon using Hep‐2 cells. Thus a negative Anti-Ds-DNA does not rule out the diagnosis of
SLE. Farr radioimmunoassay test is considered the gold standard test for the
detection of Anti-Ds-DNA antibodies and the combination of an elevated titer of Anti-Ds-DNA antibodies and a low serum C3
had a high positive predictive value for the diagnosis of SLE. Of all the Anti-Ds-DNA antibodies detection methods, the Crithidia Luciliae immunofluorescence test (CLIFT) is
thought to have the highest specificity for SLE and can be used to confirm the presence of
Anti-Ds-DNA antibodies. Changes
in Anti-Ds-DNA antibodies titers can correlate with disease activity and can
correlate with the development of lupus nephritis and can be useful in monitoring
disease activity. Indeed, high (>200 IU/mL) titers of Anti-Ds-DNA antibodies have been shown to
be an independent predictor of moderate to severe SLE flares. Low C3 has been
shown to be an independent predictor of severe lupus flare. Despite this, a
subset of patients with elevated Anti-Ds-DNA antibodies titers and hypocomplementaemia do not
demonstrate evidence of clinical disease activity when followed up. The
peripheral staining pattern of antinuclear antibody does not correlate with
anti-DNA antibodies or with disease activity. Anti-Ds-DNA antibodies can also be seen in drug-induced lupus
especially secondary to anti-TNF agents and interferon-alpha. Rarely, low
titers of Anti-Ds-DNA antibodies have been reported in rheumatoid arthritis and
Sjogren syndrome.
Several other antibodies occur in patients
with SLE. Antibodies to soluble cellular antigens include anti-Sm antibody are seen in less than 30% of SLE patients
but have 99% specificity for SLE and are included in the ACR criteria and
appears to be specific for the disease, occurring particularly in patients with
nephritis, neuropsychiatric lupus, renal disease, pulmonary fibrosis, serositis
and peripheral neuropathy, although they are not of use in monitoring overall
lupus disease activity. Anti-Smith antibodies in SLE are usually
always associated with Anti-U1-RNP antibodies which are seen in up to 30% of
SLE patients. Anti-U1-RNP antibodies can also be seen in mixed connective
tissue disease (MCTD), although in MCTD, Anti-Smith antibodies are lacking.Anti‐RNP antibodies occur
in 25% of patients with SLE. The presence of high titers of anti‐RNP antibodies is
characteristic of mixed connective tissue disease. It has been reported that
titers correlate with disease activity. Antihistone antibodies are associated
with drug‐induced lupus but are
of limited value in the diagnosis or clinical assessment of patients with SLE. Anti-Histone antibodies are not specific for
drug-induced lupus and can be seen in 50% to 70% of cases of SLE.
Anti-Ribosomal-P antibodies are very specific for SLE, although their
prevalence in SLE is less than 5%, and they may correlate with neuropsychiatric
manifestations of SLE. Anti-Ro (SSA) and Anti-La (SSB) antibodies target
ribonucleoprotein particles. Anti-Ro and Anti-La antibodies are seen in up to
90% of cases of Sjogren syndrome but can be seen in SLE as well (Anti-Ro in up
to 50% and Anti-La in up to 20%). In SLE, they may be associated with secondary
Sjogren syndrome and keratoconjunctivitis sicca, photosensitivity, congenital heart
block, and neonatal lupus.
Anti‐Ro antibody is also found
in SCLE as well as ANA‐negative
SLE patients and lupus‐like
syndromes with genetic deficiencies of C1q, C2 or C4. Anti‐Ro is an antibody to
an RNP derived from RNA polymerase III‐transcribed hY RNAs with a protein component that appears
to be the main target. Two different proteins, one of 60 kDa and another of 52
kDa, react with most positive sera. The 60 kDa protein predominates in SLE, the
52 kDa in Sjögren syndrome. Anti‐La, an antibody to another RNP product of RNA polymerase
III, is present with anti‐Ro
antibody. Anti‐Ro and anti‐La antibodies should
be tested in any female patient with SLE, mixed connective tissue disease,
Sjogren syndrome or other systemic rheumatology conditions who is planning a
pregnancy because of the increased risk of neonatal lupus syndrome. Anti-Centromere and Anti-topoisomerase-I (SCL70)
antibodies are seen in systemic sclerosis and rarely in SLE (less than 5%).
Anti-Histidyl-tRNA-synthetase antibodies are seen in myositis. Patients
with SLE may also have antiphospholipid antibodies (lupus anticoagulants,
anti-cardiolipin, and anti-beta-2-glycoprotein I antibodies) and are associated
with more thrombotic events and adverse pregnancy-related outcomes.
Although several different antibodies occur in the same
patient, they fluctuate independently, and the antibody profile may alter over
the years. The only characteristic pattern of antibody appears to occur in the
LE–erythema multiforme syndrome, in which there is a speckled type of
antinuclear factor, a specific precipitating antibody (originally designated
SjT but now thought to be anti‐La) and rheumatoid factor.
This syndrome is occasionally found in cases of SLE as well as DLE, in which it
was originally described. In cases associated with SLE, homogeneous antinuclear
factor is also usually present.
Cryoglobulins may be found in 11% of patients.
Cryoglobulinaemia may precede the manifestations of SLE by many years. Cold
agglutinins occur in 6%.
Inherited deficiencies of the major complement components
may occur in SLE, usually as autosomal recessive traits. These include C1, C2
and C4, as well as C5–C9. The most common is homozygous C2 deficiency in which
SLE occurs in approximately 30% of patients. Clinically, the lupus‐like
syndrome in C2 deficiency shows a low incidence of renal disease, but more
cutaneous involvement and arthralgia. Serum antinuclear antibodies and anti‐dsDNA
antibodies are often lower in patients with complement deficiency‐associated
SLE, compared with idiopathic SLE; however anti‐Ro
antibodies appear more frequent. Isolated C1q deficiency has also been
reported, and there is an association between C1 esterase inhibitor deficiency
and SLE. These patients may be helped by danazol. In addition to a tendency to
SLE, patients with homozygous deficiencies of C1, C2 or C4 often have a high
risk of recurrent bacterial infections.
Major Autoantibodies Associated with
SLE
Severity of disease
Disease
activity is categorized into mild forms, moderate and severe. Mild disease
forms are clinically stable with no life‐threatening organ involvement, mainly
manifesting as arthritis or mucocutaneous lesions. Patients with moderate
disease activity have more serious manifestations, such as cutaneous vasculitis
or pericarditis, and severe disease activity is defined as organ‐ or life‐threatening.
Predictors
The
most useful laboratory tests to predict a SLE flare (particularly lupus
nephritis) are an increasing serum level of anti‐DNA antibodies and a
fall in complement levels (especially C3). High levels of antibodies to complement
C1q are also associated with activity of lupus nephritis.
However,
not all patients with these serologic markers have active disease, and these
markers do not necessarily predict disease exacerbation.
It
is useful to follow tests that indicate the status of organ involvement known
to be present during SLE flares. These might include urinalysis for hematuria
and proteinuria, hemoglobin levels, platelet counts, and serum levels of
creatinine or albumin.
In
any year approximately 50–60% of patients will experience a flare, with 10% of
this group experiencing a severe flare. The leading causes of death in the
first decade of disease are systemic disease activity, renal failure,
infections and thromboembolic events. Subsequently, atherosclerosis and cancer
become more common causes of death.
Antinuclear antibody‐negative SLE
The concept of ANA‐negative lupus was
introduced in 1976 with many patients presenting to dermatologists with
cutaneous findings similar to SCLE, positive anti‐Ro
antibodies and photosensitivity. Clinically, a non‐scarring
malar flush, oral ulceration and photosensitivity, with papulosquamous or
annular lesions on the face, trunk and arms, are prominent, but arthritis,
serositis, renal disease and hematological involvement are less frequent than
expected in SLE. In approximately 5–10% of patients with SLE, antinuclear
factor cannot be demonstrated using standard substrates such as rat or mouse
liver. This is a problem in less than 2% if Hep‐2
cells are used. These patients frequently have anticytoplasmic antibodies. Over
60% of patients have anti‐Ro antibodies and approximately one‐third
have anti‐La
antibody (anti‐La
rarely occurs without anti‐Ro). Twenty‐five
per cent have antibodies to single‐stranded DNA. There
is no difference in the histology of the skin between antinuclear‐negative
and ‐positive
cases. Immunoglobulins and complement are found at the dermal–epidermal
junction in 70% of patients, but are rare in the non-light-exposed uninvolved
skin. Topical steroid therapy may be helpful, but oral antimalarials and
steroids may be required. Approximately 10% of patients eventually become
positive for antinuclear factor.
Evaluation
for systemic lupus erythematosus
ANA,
antinuclear antibodies; BUN, blood urea nitrogen; CBC, complete blood count;
CRP, C-reactive protein; ds, double-stranded; ESR, erythrocyte sedimentation
rate; Sm, Smith.
|
EVALUATION FOR SYSTEMIC LUPUS
ERYTHEMATOSUS |
|
History and review of systems |
|
Physical examination |
|
·
Specific cutaneous lesions
·
Nonspecific cutaneous lesions
·
Lymphadenopathy, arthritis |
|
Laboratory tests |
|
·
ANA with profile (anti-dsDNA, -Sm)
·
Urinalysis
·
CBC with differential, platelet count
·
Chemistries (BUN, creatinine)
·
ESR, CRP
·
Complement levels (C3, C4)
·
Anti-phospholipid antibodies |
Laboratory investigations are frequently necessary to
confirm the diagnosis. Complete blood counts, liver function tests and renal function
tests including serum creatinine, urinalysis and urine protein quantification
(24-hour urine protein, or spot urine protein/creatinine ratio) shall be
checked to assess organ involvement. Anemia, of some degree, is
found in approximately 75% of patients and is brought about by deficiency of
iron, autoimmune hemolytic anemia (AIHA), drug‐induced
myelotoxicity or renal failure. The serum iron is usually low and may rise
after corticosteroid therapy. A positive Coombs’ test can occur in the absence
of hemolytic anemia, and was present in 15% of cases. Although leukopenia
occurs in roughly 50% of patients with SLE, more specifically, lymphopenia is a
characteristic feature of the condition, leukocytosis may occasionally be
found. The platelet count is reduced in approximately 20% of cases (<100
000/mm2) and is usually below 40 000/mm2 in patients presenting with thrombocytopenic
purpura. The presence of thrombocytopenia correlates with increased morbidity
and cumulative damage. Hyposplenism may occur. The ESR is raised at some time
in nearly 90% of patients; C‐reactive protein is usually
normal in the absence of infection, however an elevated level may also indicate
disease activity so these values should be interpreted in context. Polyclonal
gammopathy is commonly observed in patients with SLE and is an indication of an
autoimmune reaction. Hypoalbuminaemia is also reported in 30–50% of patients
and the measurement of baseline immunoglobulins may help diagnose primary or
secondary immune deficiencies associated with SLE and treatment, respectively.
IgE antibodies may be raised and may correlate with disease activity including
nephritis in SLE. False positive serological tests for syphilis are found in approximately
25% of patients. Thrombosis occurs with the lupus anticoagulant, but
occasionally hemorrhage results from other hematological abnormalities such as
disseminated intravascular coagulopathy or thrombocytopenia, seen in up to 50%
of patients with CAPS. The lupus anticoagulant is one of a number of APAbs that
may be found in up to 50% of patients with SLE. As well as thrombosis, central
nervous system disease is strongly related to the presence of these antibodies.
Complements C3 and C4 shall be checked in patients with SLE or
suspicion of SLE and low complement levels indicate complement consumption and
may correlate with disease activity. Joint radiographs may demonstrate
peri-articular osteopenia, deformities or subluxation, but rarely show erosions.
Chest imaging with CT-scan, cardiac workup including echocardiography
(trans-esophageal when suspecting Libman-sacks endocarditis), CNS work up
with MRI and/or lumbar puncture shall be pursued if specific organ involvement
is suspected. Renal biopsy shall always be performed if suspicion of lupus
nephritis. Skin biopsies can be considered especially if atypical presentation.
The LE cell phenomenon, first described by Hargraves et
al., is the basis for the LE cell test, which is positive in over 80% of patients.
LE cells are neutrophils that have engulfed the nuclear material from
degenerative white cells in the presence of an antibody to
deoxyribonucleoprotein (the LE cell factor). The phagocytosed nuclear material
is homogenous and displaces the neutrophil nucleus to one side. Sometimes,
large masses of nuclear material are found extracellularly and, with
surrounding leukocytes, form rosettes. LE cells, if present in large numbers,
are highly suggestive of SLE, but the occasional LE cell is sometimes demonstrated
in other conditions, including chronic DLE.
A positive LE cell test is also a feature of drug‐induced
LE; LE cells are demonstrated in most patients with procainamide‐induced
lupus. The LE cell test has now been superseded by tests for antinuclear
factors and anti‐DNA antibodies.
Treatment
The
goal of treatment in SLE is to prevent organ damage and achieve remission. The
choice of treatment is dictated by the organ system/systems involved and the
severity of involvement and ranges from minimal treatment (NSAIDs,
antimalarials) to intensive treatment (cytotoxic drugs, corticosteroids).Treatment
is broadly divided into two:
Non-pharmacological and preventive intervention and Pharmacological therapies.
Non-pharmacological
and preventive intervention
Several
non-pharmacological measures and other medical interventions are important in
the comprehensive management of SLE, in addition to the specific medication
regimens.
Education of the patient
Patient education, physical and lifestyle measures and emotional
support play a central role in the management of SLE. Patients with SLE shall
be well educated on the disease pathology and the potential signs and symptoms
of organ involvement that may lead to early recognition and intervention which
may prevent organ damage. Further, patients shall be educated about the
importance of compliance with medications, clinical and laboratory evaluations.
Patients with SLE suffer from significant stress related to the disease and
complications and have higher rates of anxiety and depression. Stress reduction
techniques, good sleep hygiene, exercises, and use of emotional support shall
be encouraged and sometimes involvement of psychiatry may help. Dietary
recommendations shall include avoiding alfalfa sprouts and echinacea and
including a diet rich in vitamin-D.
Sun protection
Exposure to ultraviolet (UV) light may
exacerbate or induce systemic manifestations of SLE. Therefore, all patients with SLE should avoid exposure to
direct or reflected sunlight and other sources of UV light, by timing their activities appropriately. Patients should be
advised to wear broad‐brimmed
hats, light-weight
loose-fitting dark clothing covering the maximum portion of the body and using
broad-spectrum (UV-A and UV-B) sunscreens with sun protection factor (SPF)
≥55as patients
with photosensitivity may develop fatigue and disease flares following UV light
exposure. Sunscreen with avobenzone (blocks UVA‐1), titanium dioxide or zinc oxide (block UVB
and UVA‐1) are also
recommended to block longer UVA wavelengths.
Smoking cessation
Smoking
has been associated with more active disease. Smoking increases the
already higher risk of accelerated atherosclerosis in those with SLE. There is
also evidence to suggest that smoking diminishes the efficacy of
hydroxychloroquine.
Immunizations
Patients
should receive appropriate immunisations prior to the institution of
corticosteroids (>10mg/day) or immunosuppressive therapies.
Vitamin D
The
majority of patients with SLE have low serum levels of 25‐hydroxyvitamin D
(calcifediol), probably due to avoidance of sun exposure and/or use of
sunscreen products. So it is important to monitor serum 25‐hydroxy vitamin D at
baseline and to treat appropriately to ensure that recommended minimum serum
levels of 30 ng/mL (75 mmol/L) are achieved. To correct vitamin D deficiency,
1000 IU per day of oral vitamin D3 (cholecalciferol) is recommended as this is
more effective than vitamin D2. Patients with SLE are advised to take oral
vitamin D3 (cholecalciferol) 1000 IU daily PO to supplement dietary vitamin D. This
measure is important to help counter the increased risk of osteoporosis
associated with corticosteroid use.
Treating comorbid
conditions
Cardiovascular
risk factors and cardiovascular disease, pulmonary hypertension and
antiphospholipid syndrome, as well as osteopenia or osteoporosis, are among the
comorbid conditions that can be treated and for which screening tests need to
be performed. Modifiable risk factors, such as hypertension and
hyperlipidaemia, should be identified and concurrently managed.
Pharmacological therapies
The choice of therapy for SLE is highly individualized
and depends on the predominant symptoms, organ involvement, response to
previous therapy, and disease activity and severity. It is important to assess
the patient's progress by general well‐being and relief of
symptoms rather than by strict attention to laboratory abnormalities. The ESR
and DNA antibodies are variable and a poor guide to the adequacy of therapy;
the titer of ANAs often persists unchanged despite clinical remission. As
mentioned previously, anti‐dsDNA antibody and serum
complement levels may be helpful in predicting exacerbations. Despite this, a
subset of patients with elevated anti‐dsDNA titers and
hypocomplementaemia do not demonstrate evidence of clinical disease activity
when followed up. Furthermore, although there is some evidence that a return of
serological abnormalities to normal is followed by a prolonged remission,
exceptions indicate that serological data alone cannot be used as a basis of
therapy.
The use of the antimalarials chloroquine or
hydroxychloroquine should also be encouraged, not only in the management of
mild disease but in all disease subtypes because they prevent lupus flares and
increase the long‐term survival of patients. Antimalarials
have also been found to work synergistically with mycophenolate mofetil for the
treatment of membraneous nephritis and are recommended by both the ACR and the
European League against Rheumatism (EULAR) guidelines for patients with lupus
nephritis. Additionally, they have a modest effect on lipid profile,
cardiovascular disease and thrombotic risk. Hydroxychloroquine in particular
has been found to decrease lupus activity in pregnancy without harming the
baby. Quinacrine is contraindicated in pregnancy as it crosses the placenta.
Corticosteroids are useful, but dosage depends on the
degree of organ involvement and disease severity. Low‐dose
oral prednisolone (0.1–0.2 mg/kg) may be useful in patients with mild SLE and
musculoskeletal manifestations resistant to other therapies. High doses of oral
prednisolone (1–1.5 mg/kg) or IV methylprednisolone 1 g daily for 3 days (pulse
therapy) may be useful for severe disease with major organ involvement (e.g.
renal, systemic vasculitis or neurological involvement). Once the condition
appears to be under control, the dosage may be reduced until a maintenance dose
is reached, ideally <6 mg, as the Hopkins lupus cohort have demonstrated
that doses of greater than 6 mg increase the risk of organ damage by more than
50%. A single daily dose given in the morning produces fewer side effects and
does not impair the therapeutic response. Some fulminating cases have been
treated with massive doses of steroids but the advantages of such therapy
rarely outweigh the risks, and complications such as steroid‐induced
psychosis may occur, which may be difficult to distinguish from
neuropsychiatric SLE. Steroid myopathy also can occur with high‐dose
steroids, but usually improves with tapering of steroids and physical therapy.
For more severe disease, immunosuppressive drugs are used
to minimize the risk of damage and to act as steroid‐sparing
agents. When considering the choice of immunosuppressant, as well as disease
severity, the long‐term risk of malignancy must be
considered. Cyclophosphamide has been associated with bladder cancer,
myelodsplastic syndromes, hematological malignancies, cervical atypia and skin
cancers. Mesna may reduce urotoxic side effects, and monthly IV therapy is
rarely complicated by bladder injury. Azathioprine is associated with the
development of lymphomas and an increased risk of human papillomavirus‐related
premalignant and malignant lesions. Methotrexate is teratogenic and
contraindicated in women within 3 months of planned conception. Additionally,
mycophenolate is contraindicated in pregnancy and in patients wishing to become
pregnant; a transition from mycophenolate mofetil to azathioprine should be
made in the preceding months because of the teratogenic risk.
For induction therapy in lupus nephritis, pulsed IV
cyclophosphamide is preferred to oral cyclophosphamide due to reduced toxicity,
particularly bladder injury, and may be followed by mycophenolate mofetil or
azathioprine (the former may be more efficacious as maintenance therapy).
However, mycophenolate mofetil may also be used as an alternative for induction
treatment of mild to moderate lupus nephritis with similar efficacy to
cyclophosphamide.
Because of its slower onset of action, azathioprine is
often used as a steroid‐sparing agent and as a maintenance drug
following the control of more acute SLE. It has also been reported to be
effective in severe cutaneous disease and in the treatment of chronic active
hepatitis complicating lupus. Azathioprine may be used in pregnancy as there
have been no reports of teratogenicity.
Mycophenolate mofetil is the morpholinoethyl ester of
mycophenolic acid (MPA), which is a non‐competitive
reversible inhibitor of inosine‐5'‐monophosphate
dehydrogenase, a necessary enzyme in the de novo pathway of purine synthesis.
This de novo synthesis pathway is uniquely essential to activated lymphocytes.
Inhibition of both T‐ and B‐lymphocyte
proliferation, inhibition of antibody formation and prevention of leukocyte
migration by MPA activity is the result. For patients with significant
gastrointestinal side effects, which can occur in up to one‐third
of patients, titrating the dose from 500 mg twice daily to 1–1.5 g twice daily
with weekly increases may be useful. In those who are still intolerant,
switching to sustained‐release MPA may be better tolerated and
can smooth out blood levels.
Methotrexate may be a useful adjunct in patients with
mild to moderate SLE and recalcitrant mucocutaneous lesions and musculosketal
symptoms. Following the failure of
antimalarials in patients with predominant skin disease, methotrexate at doses
of 7.5–15 mg is an effective and quick acting therapy. Patients should be
monitored regularly for a decline in renal function, and their full blood
picture and liver function tests followed. In addition, meticulous
contraception is mandatory when used in women at risk of pregnancy.
Intravenous immunoglobulin may also be a useful adjunct
in resistant skin disease. A trial of 400 mg/kg/day over 5 days led to a partial
or complete remission of skin disease in 63% (10/16) of patients in one center.
Cyclosporine has been used in resistant cases and for non‐renal
lupus at a dose of 3-5mg/kg. Plasmapheresis may be useful in managing life‐threatening
complications such as fulminating vasculitis or central nervous system disease
or for renal disease that is resistant to corticosteroid or cytotoxic therapy.
Although UV light may exacerbate SLE, UVA‐1 (340–400 mm) has
been shown to have modest effects on cutaneous lesions. A low‐fat,
high‐marine‐oil
diet (eicosapentaenoic acid: Maxepa 20 g/day) modified disease activity in 27
patients in a placebo‐controlled trial over 3 months.
For patients with lupus non‐specific
skin eruptions dapsone may be useful for the treatment of urticarial lesions
and bullous eruptions.
An increasing understanding of the pathophysiology of
lupus and the recognition of the multifaceted role that B cells play in lupus,
has led to the development of novel biological drugs to treat lupus, including
rituximab (monoclonal antibody to CD20) and belimumab (a B‐lymphocyte
stimulator inhibitor). Both the ACR and EULAR support the use of rituximab in
the treatment of refractory nephritis. In a post hoc analysis of two phase III
trials (BLISS‐52
and BLISS‐76),
belimumab has also been shown to improve overall SLE activity, particularly
mucocutaneous and musculoskeletal activity at weeks 52 and 76, respectively.
Other biological therapies that may be useful in SLE include the monoclonal
antibody tocilizumab, an IL‐6 receptor inhibitor, and
the T‐cell
inhibitor abatacept, a fusion protein of the T‐lymphocyte‐associated
antigen 4 and modified Fc portion of human immunoglobulin. It is now known that
an increased serum concentration of IFN‐α is associated with
a distinct IFN signature in the peripheral blood and increased disease
activity. In view of this, there are ongoing studies investigating inhibitors
of this pathway including the monoclonal antibodies that target IFN‐α,
sifalimumab and rontalizumab.
Cutaneous manifestations
For skin disease alone, topical
therapy is an appropriate first line treatment for individual lesions. Potency
depends on the site, but generally potent fluorinated steroids should only be
used for short periods on very inflammatory lesions on the face because of the
risk of atrophy and telangiectasia. Alternatively, the calcineurin inhibitors
tacrolimus and pimecrolimus may be equally efficacious. Intralesional triamcinolone acetonide (2.5–5
mg/mL) may also be useful for individual lesions, particularly on the scalp.
For patients with extensive or resistant cutaneous
disease, antimalarials are used. Hydroxychloroquine
is the drug of choice for most cutaneous manifestations and very efficacious.
Quinacrine can be used if intolerance or adverse effects of hydroxychloroquine.
Methotrexate can be used if no response to hydroxychloroquine. For severe
or resistant disease, systemic corticosteroids, mycophenolate mofetil, and
belimumab can be considered. Other alternatives include thalidomide, cyclophosphamide,
IVIG and rituximab.
For patients with lupus non‐specific
skin eruptions dapsone may be useful for the treatment of urticarial lesions
and bullous eruptions.
Musculoskeletal
manifestations
Hydroxychloroquine
is the initial drug of choice for lupus arthritis. If no response, methotrexate
or leflunomide can be considered. Belimumab and rituximab can be considered in
refractory cases. Symptomatic therapy for joint pain using non‐steroidal
anti‐inflammatory
drugs is valuable.
Hematological
manifestations:
Drug-induced cytopenias shall be excluded. Mild cytopenias usually
require no treatment. For moderate to severe cytopenias, corticosteroids are
the mainstay of treatment, and azathioprine or cyclosporine-A can be used as a
steroid-sparing agent. Severe refractory cytopenias may require intravenous
pulse dose steroids, mycophenolate mofetil, rituximab, cyclophosphamide,
plasmapheresis, Recombinant G-CSF or splenectomy.
Cardiopulmonary
manifestations
Serositis usually responds to NSAIDs or moderate to high dose oral
corticosteroids. Hydroxychloroquine and methotrexate can be considered as
steroid-sparing agents. Acute lupus pneumonitis requires high dose IV pulse
corticosteroids while plasmaphereses and/or cyclophosphamide may be needed if
diffuse alveolar hemorrhage present. Interstitial lung disease can e managed by
low to moderate dose corticosteroids with immunosuppressive agents such as
azathioprine or mycophenolate mofetil. Pulmonary arterial hypertension requires
vasodilator therapy, while thrombotic complications such as pulmonary embolism
require anticoagulation. High-dose corticosteroids are required for the
management of myocarditis and coronary arteritis.
CNS manifestations
Accurate diagnosis, and ruling out other potential causes is critical
before initiating treatment for neuropsychiatric manifestations of SLE. High
dose corticosteroids with immunosuppressive agents such as cyclophosphamide,
azathioprine, or rituximab are used for inflammation-related neuropsychiatric
manifestations such as optic neuritis, aseptic meningitis, demyelinating
disease, etc. Lifelong warfarin is indicated in cases of thromboembolic CNS
events associated with antiphospholipid antibody syndrome. High dose
corticosteroids can be used in cognitive impairment, although there is no
robust data on this.
Renal manifestations
Lupus nephritis (LN) shall be confirmed with a biopsy which serves
not only to confirm the diagnosis, but also to rule out other causes, and helps
to classify the disease. Class I and II LN shall be treated with the
Renin-angiotensin-aldosterone system blockade. Immunosuppression with high dose
corticosteroids followed by azathioprine is indicated only if proteinuria more
than 1 gram/day. Membranous LN (Class V) shall also be treated with the
Renin-angiotensin-aldosterone system blockade. If proteinuria of more than 1
gram/day is present (which is frequent in Class V LN), induction therapy with
high dose corticosteroids and azathioprine (mild disease) or cyclosporine
A/mycophenolate mofetil/IV cyclophosphamide (moderate to severe disease)
followed by maintenance therapy with azathioprine, mycophenolate mofetil or
cyclosporine-A shall be used. Corticosteroids shall be gradually tapered during
maintenance therapy. Proliferative LN (Class III/IV) requires more aggressive
therapy. Induction therapy is with IV pulse dose methylprednisolone followed by
high dose oral steroids in combination with mycophenolate mofetil, IV
cyclophosphamide or azathioprine (only in mild disease). Maintenance therapy
with mycophenolate mofetil or azathioprine shall be continued for at least 3
years. IV pulse cyclophosphamide for 1 year can be considered as maintenance
therapy for severe disease. Lupus nephritis patients need very close monitoring
of their renal function and proteinuria in addition to other SLE disease
activity markers. Flares and incomplete remission are common. Renal replacement
therapy and transplant may be needed in some patients.
Pregnancy
manifestations
Pregnancy
shall be considered only if the disease was quiescent at the time of and 6
months prior due to an increased risk of flares otherwise. Contraception if
needed shall be used until then and shall be progesterone-only contraception.
Hydroxychloroquine
is considered safe in pregnancy, has been associated with a significant
reduction in flares and disease activity, and may decrease the risk of
recurrent cardiac neonatal lupus and heart block in mothers with anti‐Ro
antibodies
and shall be continued through pregnancy. Azathioprine and low dose corticosteroids
can be used for mild manifestations. Oral corticosteroids are
relatively safe in the treatment of lupus during pregnancy. The dosage of
corticosteroids should be temporarily increased at the time of delivery and
postpartum. Corticosteroids do not appear to cause impairment of growth or
malformation in the fetus, and, as in women who are not pregnant, an increased
risk of maternal hypertension and diabetes, but high-dose steroids during early
pregnancy can cause cleft palate. Babies of mothers with untreated SLE are
usually smaller than expected, and corticosteroids may assist growth of the
fetus in utero. Prednisolone is recommended, as less than 10% of the dose will
cross the maternal–fetal membranes. If the patient is on azathioprine, this
should be continued as there is no evidence of an increase in the malformation
rate. However, mycophenolate mofetil is contraindicated and it is recommended
that women transfer to an alternate immunosuppression prior to conception, such
as azathioprine.
Other immunosuppressive agents including methotrexate,
leflunomide, and cyclophosphamide are teratogenic and contraindicated in
pregnancy. Rituximab and belimumab shall also be avoided during pregnancy.
Patients with antiphospholipid antibody syndrome shall be transitioned from
warfarin to low-molecular-weight heparin and aspirin before pregnancy. For
females with positive Anti-Ro or Anti-La antibodies with a history of neonatal
lupus in previous pregnancy close fetal heart monitoring with weekly or
alternate-weekly fetal echocardiography is recommended during the second
trimester. First or second-degree heart block shall be promptly treated with
dexamethasone, although prophylaxis with dexamethasone is not recommended. A
complete heart block is irreversible and requires a permanent pacemaker in the
infant.
Although traditionally it was recommended that estrogen‐containing
contraceptives, even at low dosage, should be avoided in women with SLE,
clinical trials have now shown that the use of oral contraceptives in women
with stable disease does not increase the risk of flare. However, estrogen‐containing
contraceptives should be avoided in lupus patients with positive
anticardiolipin ± lupus anticoagulant. Conversely, it is recommended that
patients with SLE wishing to commence this method of contraception should be
screened for these antibodies. If mechanical methods of contraception or
intrauterine devices are not possible, then progesterone‐only
contraceptive Depo‐Provera is an alternative option,
although its use for more than 2 years may increase the risk of osteoporosis.
In patients taking azathioprine no adverse events have
been reported in breastfed infants exposed to the drug, but a small study
showed that the majority of azathioprine is excreted within 4 h of ingestion,
prompting the recommendation that feeds be given at least 4 h post maternal
dose. Breastfeeding is probably safe if the patient is on aspirin, low‐dose
steroids or hydroxychloroquine, but should probably be avoided if other
immunosuppressives are used.
Other management
considerations
Hydroxychloroquine shall be used in all patients with SLE given
its benefits beyond just the management of active manifestations including
anti-thrombotic properties, and preventing flares. Patients on hydroxychloroquine
will require regular ophthalmology exams to monitor for the rare but
irreversible maculopathy associated with this drug. Corticosteroids are very
frequently used in SLE, with many patients unable to completely taper them.
Long term adverse effects of corticosteroids shall be considered and monitored
for including osteoporosis, glaucoma, cataract, and avascular necrosis.
Patients on high dose corticosteroids will also need antibiotic prophylaxis to
prevent infections. Most immunosuppressive agents used in SLE have several
potential adverse effects ranging from cytopenias and hepatotoxicity with most
to an increased risk of urinary bladder cancer with cyclophosphamide. These
patients shall be appropriately and closely monitored for adverse effects of
these agents.
Lupus is a chronic inflammatory disorder with no cure. It can
affect many organs and leads to a very poor quality of life without appropriate
management. Premature death is common from a variety of causes. To reduce
morbidity and mortality, an inter professional team should educate and
manage patients with SLE.
The primary care provider and nurse practitioner should educate
the patient on avoiding triggers that cause flare-ups. In addition, the patient
should be told to avoid UV light and minimize exposure to the sun. When going
out, appropriate garments, sunglasses, and a wide brim hat are recommended. The
dietitian should educate the patient on the importance of a low-fat diet to
prevent hyperlipidemia. In addition, because patients with lupus are told to
avoid the sun, vitamin D supplements are recommended. The physical therapist
should educate the patient on the importance of exercise. The pharmacist
should educate the patient on the importance of medication compliance and
avoiding smoking. The nurse practitioner should counsel the patient on family
planning and contraception. Many drugs used to treat are teratogenic, and thus,
contraception is highly recommended. Multispecialty involvement is often
needed in managing and monitoring SLE. While the involvement of a
rheumatologist is crucial, other specialties including dermatology, cardiology,
neurology, pulmonology, ophthalmology, nephrology, gastroenterology and
gynecology may be needed. Close communication between the providers and the
patient and family, and consideration of patient preferences while deciding
therapy is strongly recommended.