Alopecia
areata
Salient
features
·
Alopecia
areata is a non-cicatricial hair loss in any hair-bearing area of the body with
a multi-factorial, autoimmune pathogenesis, and an unknown etiology
·
It
occurs in both genders equally and can affect every age group, although incidence
at in younger age groups is higher.
·
It
is the most common form of hair loss in children. Clinically, it presents with
well-demarcated round or oval bald spots on the scalp or other parts of the
body.
·
Of
patients with alopecia areata, 5% develop hair loss of their entire scalp hair
(alopecia areata totalis) and 1% develops alopecia areata universalis (loss of
total body hair).
·
Nail
changes include geometric pitting or sandpaper nails (trachonychia)
·
Alopecia
areata is thought to be an autoimmune disease with a possible hereditary
component.
·
In
general, alopecia areata is a medically friendly condition, but it can coexist
with other autoimmune disorders such as Hashimoto thyroiditis and vitiligo.
Introduction
Alopecia
areata is a relapsing and remitting, chronic inflammatory disease that involves
the hair follicle and sometimes the nails. It is a CD8+ T-cell –mediated, organ
specific (hair-specific) autoimmune non-cicatricial alopecia that is occurring
in genetically predisposed individualstriggered by some yet unknown environmental
factors.
AA
can undergo spontaneous remission. As the current episode duration is an
important prognostic indicator, AA is categorised as acute (<12 months) or
chronic (>12 months).
In the
acute progressive stage of alopecia areata, T lymphocytic infiltrates are seen
around, and sometimes within, the hair bulb region of anagen follicles, which has
been described as a “swarm of bees”. These cytotoxic CD8+ NKG2D+ T
cells, which produce interferon (IFN)-γ, are
thought to play a key role in pathogenesis.This inflammation leads to
increased number of catagen hairs and finally to dystrophic, miniaturized
hairs. Despite the presence of this inflammatory infiltrate, the follicle
retains its potential to produce hair, implying that the follicular stem cells
remain viable.
The chronic relapsing nature of
alopecia areata and its profound effect on physical appearance make the
development of this condition a distressing and life-changing event for many
affected individuals.
Epidemiology
The average lifetime risk of developing alopecia
areata is estimated to be 1-2%. AA may
appear at any age, but as many as 60% of patients with AA will present with
their first patch before 20 years of age, and
80% before the age of 40 years.
AA affects both sexes
with equal frequency; however men are afflicted with the severe forms of the
disease more often.
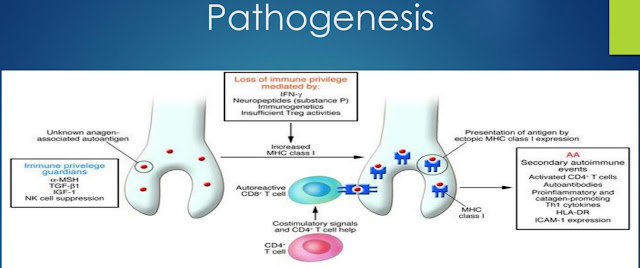
Pathogenesis
Pathogenesis of alopecia areata
The
pathogenesis of AA remains incompletely understood to date, although a complex
interplay between loss of immune privilege in the hair follicle,
autoimmune-mediated hair follicle destruction and up regulation of inflammatory
pathways has been advocated to explain the development of this condition. Auto reactive
CD8 and CD4 T cells infiltrate the hair follicles and attack hair
follicle-derived auto antigens while sparing the stem cell compartment.
There
are multiple hypotheses regarding the pathogenesis of alopecia areata, ranging
from an environmental insult such as a viral infection to a genetic
susceptibility to T-cell-driven autoimmunity. The latter could result from a
primary attack of the immune system against the hair follicle or a breakdown in
the hair follicle’s immune privilege which leads to a secondary attack by the
immune system (s. Of course, it is most likely that there is interplay of
several factors and depending upon the particular patient or subtype, one
factor may play a more important role.
Evidence
in support of a T-cell-driven autoimmune process includes the observation that
CD8+ T cells are the initial intrafollicular
lymphocytes to appear in alopecia areata. Cytotoxic CD8+ NKG2D+ T cells,
which produce interferon (IFN)-γ, are thought to
play a key role in pathogenesis. Involvement of IFN-γ and
the γ-chain cytokines (IL-2, IL-7, IL-15, and
IL-21) implicates downstream signaling via the JAK (Janus kinase)/STAT pathway.
These findings represented the rationale for cytokine-targeted therapy and
indeed the JAK inhibitors tofacitinib and ruxolitinib did promote
hair regrowth in clinical studies.
Under the genetic backgrounds,
some triggers, such as viral infection, trauma, physical/emotional stresses,
activate auto reactive CD8 + T cells and Th1 cells that produce IFN-gamma.
IFN-gamma up regulates CXCL10 expression and IL-15 production with its chaperone
receptor IL-15R from hair follicle keratinocytes (outer root sheath). IFN-gamma
also collapses hair follicle immune privilege that exposes auto antigens to
auto reactive CD8 + T cells. Th1 and Tc1 cells show chemotactic activity toward
CXCL10. Effector cell is NKG2D + CD8 + T cells in AA pathogenesis.
Collapse of the hair
follicle’s immune privilege may play a role in the pathogenesis of alopecia
areata which leads to a secondary attack by the immune system. Normally, anagen
hair follicles express low levels of MHC class I molecules (thereby minimizing
presentation of auto antigens), up regulate immunosuppressant molecules such as
TGF-β1 and α-MSH, and do not activate natural killer (NK) cells by
expressing UL16 binding protein 3, an NKG2D ligand. In that hair matrix immune
privilege is restricted primarily to the anagen phase, it has been postulated
that an auto antigen from melanocytes, which are active during the anagen
phase, could be responsible for initiating the immune response against the hair
follicle.
However,
if hair follicle immune privilege (HF-IP) is collapsed by stressors, auto
antigens are revealed, resulting in an autoimmune reaction by NKG2D + CD8 +
auto reactive T cells that causes the unique pathological feature called
"swarm of bees" in the acute phase of AA. Th1 cytokines, as
represented by interferon (IFN)-γ, are dominantly detected in AA lesions and
may induce the collapse of HF-IP, including the up- regulation of MHC class I.
Heredity also plays a
part. Overall, nearly 25% of patients have a positive family history. Patients
with “early onset, severe, familial clustering alopecia areata” have a
significant association with the HLA antigens DR4, DR11, and DQ7. A family history of alopecia areata is more common in those
with disease onset before the age of 30 years. The “later
onset, milder severity, better prognostic” subsets of patients have a lower
frequency of familial disease and do not share these HLA antigens.
Alopecia areata
causes a disturbance in the normal dynamics of the hair cycle. Anagen follicles
are precipitated into telogen.
Follicles are able to
re-enter anagen but, whilst the disease is active, are unable to progress
beyond the anagen 3–4 stage. It has been suggested that they then return
prematurely to telogen and that these truncated cycles continue until disease
activity wanes.
The inflammatory
infiltrate in alopecia areata is concentrated in and around the bulbar region
of anagen hair follicles. The sparing of white hair sometimes seen in alopecia
areata has also raised the possibility that alopecia areata is primarily a
disease of hair bulb melanocytes.
Proposed pathodynamic changes in alopecia areata.
An inflammatory attack on anagen follicles precipitates follicles into telogen.
Follicles re-enter anagen but development is halted in anagen 3–4 and follicles
return to telogen prematurely.
The
onset or recurrence of hair loss is sometimes triggered by:
o
Viral infection
o
Trauma
o
Hormonal change
o
Emotional/physical stressors
Clinical features
AA
can present with a wide range of clinical heterogeneity, including a very
sudden and dramatic hair loss.
Most patients have no
symptoms, and a bald patch is noted incidentally, often discovered by a
hairdresser, by a parent, or friend. Other patients describe a burning, prickly
discomfort in the affected areas—this is known as trichodynia. Alopecia areata is conventionally classified as patchy
alopecia, alopecia totalis and alopecia universalis.
Patchy alopecia areata
Patch
alopecia areata can affect any hair-bearing area, most often the scalp,
eyebrows, eyelashes and beard.
Patchy
alopecia areata has three stages.
Sudden loss of hair
Enlargement of bald patch or patches
Regrowth of hair
Alopecia areata commonly presents
as sudden onset of one (unifocal) or more (multifocal), discrete, oval
or round, non-scarring, well-circumscribed, totally bald patches with a smooth
surface that enlarge in a centrifugal pattern. Often the
patches are from 1 to 5 cm in diameter. A few resting hairs may be found within
the patches. The
skin within the bald patch appears normal or slightly reddened. The follicular
openings are preserved and there is no atrophy. In the acute stages, gentle
pulling from the periphery of bald areas will yield more than 10 hairs. AA
typically presents on the scalp in 90% of cases, most commonly involving the
occipital scalp. Approximately half will present with a solitary lesion.
Characteristic hallmarks of alopecia areata are “black dots” (cadaver hairs, point noir), resulting from hair that breaks before it reaches the skin surface. Short, 3-4 mm in length, easily extractable broken hairs, known “exclamation point” hairs (i.e. distal end broader than the proximal end, with a blunt distal end and taper proximally) can often be seen, particularly at the margins of the bald patches during active phases of the disease when the broken hairs (black dots) are pushed out of the follicle. Microscopy shows a thin proximal shaft and normal caliber distal shaft. Exclamation mark hairs occur only in acute forms of alopecia areata and are not seen in patients with long-standing areas of hair loss. Another feature is the presence of Caudability hairs- a kink in the normal looking hairs, at a distance of 5-10 mm above the surface, when the hair is forced inwards or bent.
Regrowth
is often at first fine and white, they may be curly when previously straight,
but usually the hairs gradually resume their normal caliber and color within a few weeks or months.
It may
take months and sometimes years to regrow all the hair.
In
exceptional cases where re-growing hairs remain non-pigmented the possibility
of concurrent vitiligo should be considered.
Diffuse alopecia areata
Diffuse
AA is described as a unique AA in
which no patchy areas of hair loss are present and instead, demonstrates widespread scalp hair
thinning and increased serum IgE level. In diffuse
AA, hair thinning is acute and diffuse throughout the whole
scalp. This reduced hair density can be more evident on androgen-dependent
areas of the scalp. Prognosis is generally favorable,
especially as compared to other variants of alopecia areata, namely, alopecia
areata totalis, universalis and ophiasic areata.
Diffuse
AA is more common in patients
under the age of 40, especially in those from 20 to 40 years of age with a
strong female predominance.
Dermoscopic
signs are characteristic of AA and consist of broken hairs, black dots, yellow
dots, newly-grown short hairs, short vellus hairs, and exclamation mark hairs.
Histopathology of scalp biopsies of diffuse AA
showing typical features of AA which consists of increased proportion of
catagen and telogen hair follicles, and more intense infiltration comprising of mononuclear
cells, eosinophils, and T lymphocytes (CD3 + , and
CD8 + T) cells around hair bulbs in diffuse AA.
Hypersensitivity
may be involved in the pathogenesis of diffuse AA and the acute onset of hair
loss may be related to a relatively more intense local inflammatory
infiltration of hair loss region and an increase in serum IgE level.
Eyelashes AA
Hair
loss may also occur exclusively in the eyelashes. Eyelash AA affects young
female patients more than males and is seen more commonly in the upper than
lower lashes. The main differential in these patients is trichotillomania.
Alopecia totalis (AT) and
Alopecia universalis (AU)
Alopecia
totalis & alopecia universalis AT, hair loss of the entire scalp, and AU,
hair loss of the entire scalp and body are more severe forms of AA. An
estimated 7% of patients with AA can develop AT or AU during their clinical
course. When considering AT and AU, it is helpful to distinguish the age at
initial onset, as clinical characteristics vary based on this. Patients with an
early onset of AT or AU are more likely to have a family history of AA, nail
dystrophy or a history of concomitant medical disorders than those with late
onset.
Reticular variant
Reticular type has a
net-like pattern with multiple active and regressing patches, in which the
patient experiences hair loss in one area while at the same time undergoing
spontaneous regrowth in other sites.
Ophiasis
Ophiasis
is continuous band-like hair loss in parietal, temporal, occipital regions. It
is associated with poor prognosis.
Sisapho
The very rare ophiasis inversus, also called sisaipho type, is just the
clinical opposite of ophiasis, presents with hair
loss in the central scalp, resembling androgenetic alopecia.
Canities subita
An
intriguing feature of alopecia areata is the sparing of white hairs. In
patients with grey hair, which is an admixture of pigmented and non-pigmented
hair, the disease process appears preferentially to affect pigmented hair, so
that non-pigmented or white hair is spared by the inflammation and remain on
the scalp while dark hair falls out very quickly and is probably the
explanation for people ‘going white overnight’ in diffuse alopecia areata. The sudden
loss of pigmented hair is considered an acute episode of AA.
Alopecia areata of the nails
The
nails are an important component of the clinical picture when considering a
diagnosis of AA. Nail involvement can frequently be seen in patients with AA,
with a reported incidence ranging from 7 to 66%. Nail changes can involve one
nail, several nails or all 20 nails. Nail changes are reportedly more common in
severe patterns of AA, especially in
long-standing cases with extensive involvement.
The
most common change is geometric pitting, characterized by diffuse, uniform,
fine superficial pitting of the nail plate, i.e.
pitting in organized transverse or longitudinal rows
giving the nail a "hammered brass" appearance, which is seen in
approximately 28-34% of patients with AA. Punctate leukonychia is also common and
the distribution of white spots is usually geometrical as in pitting.
Trachyonychia (sandpaper-like roughness) or rough nails, is characterized by
brittle, thin nails with excessive longitudinal ridging and has been shown in
an estimated 3-12% of patients with AA. Onychomadesis, or complete nail
shedding from the proximal portion of the nail, can be associated with acute AA.
Of note, nail changes are not as significant in the more diffuse variant AA
incognito.
|
ALOPECIA AREATA: DISEASE AND
GENETIC ASSOCIATIONS |
|
Associated diseases |
|
·
Atopy (allergic rhinitis, atopic
dermatitis, asthma); >40% in some studies ·
Autoimmune thyroid disease (e.g. Hashimoto
thyroiditis), vitiligo, inflammatory bowel disease ·
Autoimmune polyendocrinopathy syndrome type
1 (autosomal recessive; due to mutations in the autoimmune regulator gene [AIRE]; up to 30% of patients have alopecia areata) ·
Type 1 diabetes increased in relatives of patients with alopecia areata |
|
HLA associations |
|
·
HLA-DQB1*0301 (DQ7), HLA-DQB1*03 (DQ3), and
HLA-DRB1*1104 (DR11); HLA-DQB1*03 appears to be a susceptibility HLA marker
for all forms of alopecia areata, whereas the HLA alleles DRB1*0401 (DR4) and
HLA-DQB1*0301 (DQ7) are considered markers for severe longstanding alopecia totalis/universalis |
Comorbid autoimmune disorders
AA
is thought to be an immune-mediated disease of the hair follicle and frequently
occurs in association with other autoimmune diseases, including thyroid
disease, celiac disease, pernicious anemia, Addison's disease, vitiligo, lupus
erythematosus, atopic dermatitis and allergic rhinitis, rheumatoid arthritis and
myasthenia gravis. Approximately 38% of patients with AT or AU have comorbid
autoimmune disorders; the most common of these is atopic dermatitis.
AA
is frequently associated with thyroid autoimmunity, including Hashimoto
thyroiditis and Grave's disease, with an incidence between 8 and 28%. AA may be
the only clinical manifestation of celiac disease and it has been suggested
that screening for the antibodies found in celiac disease (antigliandin and
antiendomysial) is warranted in the work-up of patients with AA.
There
is a higher prevalence of vitiligo in patients with AA. It is postulated that
both AA and vitiligo are caused by an autoimmune response targeted to hair
follicle and melanocyte antigens, respectively. This reported association has
even been shown to co localize within the same lesion.
Regarding
atopy, it is seen more commonly in patients with AA or their first-degree
relatives than the general population, regardless of AA subtype or extent. In
fact, atopic diseases, including asthma, atopic dermatitis and hay fever, are
associated with 10-60% of AA patients. Of note, AA patients with atopy have
been reported to have a higher incidence of ophiasis pattern of hair loss and
nail involvement.
Rheumatoid
arthritis is found to be associated with AA in 0.9% of patients in one study.
AA is a non-motor symptom that can be found in patients with myasthenia gravis
with an incidence ranging from 0.3 to 3%. Of note, in patients with myasthenia
gravis with AA and thymoma, there are reports of improvement of AA following
thymectomy.
Patients
with AA typically have a history of more autoimmune diseases in their family as
well. However, having more than one atopic or autoimmune disease does not place
patients at an increased risk of AA.
Comorbid genetic abnormalities
Down
syndrome has been associated with an increased incidence of autoimmune
disorders, including AA. The incidence of AA association with Down syndrome has
been reported between 1 and 11%.
AA
affects about one-third of patients with autoimmune polyendocrine syndrome type
I (APS I), an autosomal recessive disorder with organ-specific autoimmunity and
ectodermal features. APS I appear between 3 and 30 years of age and patients
often have more severe forms of AA, namely AU or AT.
Piliannulati,
which is an autosomal dominant hair disorder characterized by alternating light
and dark bands of the hair shaft, has been reported to be concomitant with AA.
However, based on current data, a direct association between piliannulati and
AA seems unlikely.
Diagnostic studies
In
many cases, the clinical characteristics of AA can lead to a swift diagnosis.
However, in some cases the clinical diagnosis may not be straightforward and
more advanced diagnostic studies may be necessary. In these cases, less
invasive diagnostic options include wash test, pull test, trichogram and scalp
dermoscopy, or more invasively a biopsy with histopathology examination may be
utilized.
Wash test
For
the wash test, patients collect hairs shed during standardized washing for quantification
and analysis. Dystrophic, anagen hairs may be present among the shed telogen
hairs in patients with presumed telogen effluvium (TE) who may in fact have AA
incognita. In AA incognito, there have been reports of greater than 100 hairs
shed daily. The modified wash test may produce from 350 to 800 terminal telogen
hairs, with dystrophic hairs comprising only a very small subset of 2-3% of
collected hairs.
Pull test
Pull test can be performed
to assess disease activity; six hairs or more shed from the periphery of the
lesion positively correlates with the disease activity. During
the pull test, gentle traction is applied to a small group of approximately
40-60 hairs to assess for hair shedding. Hairs are then examined under the
microscope. In acute AA, the pull test is very positive with extraction of
telogen and dystrophic broken roots. Dystrophic hairs are indicative of a
disease in which mitotic activity of anagen follicles has been interrupted,
such as AA and systemic chemotherapy. In active AA, hairs in the periphery of
the patch of alopecia can be easily extracted.
In
AA incognito the pull test is positive. Typically, the clinician will find
shedding telogen roots at different degrees of maturation. These are
predominantly early telogen roots, which are characterized by an epithelial
envelope surrounding the club hair. The dystrophic, broken hairs previously
mentioned in acute AA are usually absent.
Trichogram
Trichogram is a semi-invasive tool examining
the ratio of hair in various hair growth cycle phases. About 60-80 hairs are
plucked with a rubber covered haemostat after the patient has not washed their
hair for 5 days. The hair bulbs and their roots are taped to a slide for
evaluation of the hair type and anagen to telogen ratio. This study is crucial
for the diagnosis of loose anagen syndrome which has a predominance of anagen
hairs (>70%) with distorted bulbs, absent root sheaths and ruffling of the
cuticles. These findings contrast with the trichogram findings for AA, which
includes telogen and dystrophic roots.
Scalp dermoscopy
Scalp dermoscopy also known as trichoscopy
visualizes many useful patterns that are indicative of a diagnosis of AA,
including yellow dots, cadaverized hairs or 'black dots', broken hairs or
micro-exclamation mark hairs. Hair follicle ostia can be visualized easily. Of
note, loss of follicular openings indicates scarring alopecia, and excludes a
diagnosis of AA.
Overall, the most
sensitive markers for diagnosing AA are yellow dots and short vellus hairs
(shorter than 10 mm), while the most specific markers are tapered hairs with
dark thick broken tips. Black dots, micro-exclamation mark hairs and tapered
hairs correlate with AA disease activity. Black dots, yellow dots and short
vellus hairs (shorter than 10 mm) correlate with AA severity. Dermatoscopic
finding associated with a good prognosis include the transformation of vellus
into terminal hairs (increase in proximal shaft thickness and pigmentation).
Dermoscopy can also aid the clinician in finding the optimal biopsy location.
Scalp biopsy
A 4-mm punch biopsy
is standard and can be used for a more definitive diagnosis in doubtful cases.
Both horizontal and vertical sections should be evaluated.
Histopathologic
features on horizontal sections of AA depend on the stage of the lesion and
disease duration. This can be divided into early active (acute and subacute)
and longstanding (chronic) stages.
Inflammation is a
hallmark of active AA. Anagen follicles at the margins of expanding patches of
alopecia areata characteristically show a peribulbar inflammatory cell
infiltrate, composed of activated CD4+ and CD8+ T cells, together
with macrophages, langerhans cells and cells expressing NK cell markers,resembling
a 'swarm of bees' that may invade the follicular epithelium and matrix, and
even extend into fibrous tracts. Active areas are more likely to demonstrate
dilated follicular infundibula, intrabulbar infiltrate, reduction in
anagen/telogen ratio and increased terminal catagen and telogen hairs.
Later, in the chronic
phase, increased numbers of miniaturized, vellus-like hair follicles as well as
many fibrous streamers with lymphoid cells or loss of pigment may be seen.
Inflammatory infiltrate may also involve miniaturized hairs.
Eosinophils have been
noted in the peribulbar inflammatory infiltrate and fibrous tracts in all
stages of AA. Melanin has also been noted in fibrous streamers. Pigment casts
within follicular canals and follicular mucinosis have also been described.
Fibrous streamers are birefringent negative for collagen. Therefore, polarized
light may be useful to distinguish between the fibrous streamers seen in
chronic non-scarring AA and the follicular scars in scarring alopecia.
An absence of the
classic inflammation in AA has been reported, which can cause particular
difficulty in diagnosing AA. Therefore, in the setting of a lack of lymphocytic
infiltrate, other diagnostic clues include: increased number of terminal
catagen/telogen follicles, miniaturization of follicles, pigment casts within
the hair canal and eosinophils, melanin or lymphocytes in fibrous streamers.
In contrast to the
inflammatory scarring alopecias, little or none of the inflammatory infiltrate
is seen around the isthmus of the hair follicle, the site of hair follicle stem
cells. This may explain why follicles are not destroyed in alopecia areata.
An
exclamation-mark hair: pathognomonic of alopecia areata.
Types
of Alopecia Areata and Their Clinical Presentations
Panel A
shows the most common presentation of alopecia areata, with typical round areas
of complete hair loss in normal-appearing skin; multiple alopecic patches may
coalesce.
Panel B
shows alopecia areata totalis, characterized by the complete loss of scalp
hair, and
Panel C
shows alopecia areata universalis, characterized by the complete loss of
body hair.
Panel D
shows a condition known as ophiasis, in which hair loss in the occipital scalp
skin is highly resistant to therapy.
Panel E
shows a diffuse variant of alopecia areata characterized by the loss of hair
over a large scalp area, without bald patches.
Panel F
shows the phenomenon of overnight greying, which in some cases represents
massive, diffuse alopecia areata of rapid onset. Since only the pigmented hair
follicles are attacked, pre-existing gray or white hair becomes demasked.
Characteristic
Clinical and Dermoscopic Features of Alopecia Areata
Panel A
shows the characteristic features of alopecia areata in a father and his child.
The father’s hair loss occurred in well-circumscribed patches of clinically
uninflamed, symptomless skin in the beard region. The child has alopecia areata
totalis.
Panel B
shows “exclamation-mark hair,” in which the distal segment of the hair shaft is
broader than its proximal end, and
Panel C
shows “cadaver hairs” (comedo-like black dots).
Panel D
shows nail pitting, one of several nail changes that can be present in alopecia
areata, another being onychodystrophy.
Panel E
shows the regrowth of white hair shafts (poliosis) in an alopecic lesion.
Clinical diagnosis of AA became considerably easier with the development
of scalp dermoscopy. Dermoscopy allows confirmation of the diagnosis in
doubtful cases and provides criteria to assess disease activity and severity.
With dermoscopy the clinician can evaluate the whole scalp and predict whether
the disease is spreading or is stable. This is very important for therapeutic
decisions. In addition, dermoscopy aids in the evaluation of treatment efficacy
as it permits visualization of initial regrowth that is not easily visible to
the naked eye. The role of pathology in the diagnosis of AA is greatly reduced
with the advent of dermoscopy.
Differential diagnosis
The diagnosis can almost always
be made clinically. Clinical features, such as shape and
look of the patches, presence of exclamation point hair, nail changes (pitting
or sandpaper nails) and absence of scarring lead
to the diagnosis of alopecia areata. Moreover, positive family history and/or
the presence of associated diseases may give further evidence in cases of
doubt. In the acute phase, a peribulbar lymphocytic infiltrate, which has been described
as a “swarm of bees” may be found. Since
20% patients have abnormal thyroid function, it is reasonable to check this
aspect, but restoring normal function does not always cure alopecia areata.
Non-scarring AA is a non-scarring form of
hair loss and may mimic other non-scarring alopecias, including AGA, TE,
alopecia syphilitica, trichotillomania and tinea capitis.
Androgenetic alopecia and Telogen effluvium
Diffuse AA can
be misdiagnosed as other diffuse alopecia because it lacks the characteristic
patches of AA, such as telogen effluvium (TE) and androgenic alopecia (AGA)
Dermoscopy and pathological examination are of great value in diagnostic work.
All the dermoscopic and pathological features can be found in diffuse AA
patients, such as broken hairs, black dots, and exclamation mark hairs and
mononuclear cells, eosinophils, and T lymphocytes cells around hair bulbs in
diffuse AA. However, TE and AGA lack all these features and can be excluded by
pathologic findings, i.e., a terminal/vellus ratio of <4:1 without peribulbar
lymphocyte infiltration is diagnostic of AGA, whereas the ratio >7:1
suggests TE.
Alopecia syphilitica
Alopecia syphilitica,
which occurs in approximately 4% of patients with secondary syphilis, should be
considered in the appropriate clinical setting. Clinically, alopecia
syphilitica has been described as having a non-inflammatory 'moth eaten'
appearance and less commonly can present as 'essential alopecia' with diffuse
hair loss or a combination of both. Hair loss is non-inflammatory. Diagnosis
can be confirmed with syphilis serology testing and occasionally spirochetes
can be visualized in the hair follicles.
Alopecia neoplastica
Alopecia neoplastica
may be induced by tumor metastasis to the scalp and may resemble AA, even
though the scalp usually shows some signs of inflammation. Alopecia neoplastica
must be considered in patients with underlying neoplasms, especially breast
cancer in older women. Extramammary Paget's disease has also presented as
poorly circumscribed, erythematous plaque with patchy alopecia on the scalp.
Histopathologic examination of the scalp often reveals the underlying neoplasm
and aids in diagnosing alopecia neoplastica.
In children the main
sources of diagnostic difficulty are tinea capitis and trichotillomania.
Tinea capitis
Tinea capitis is a
contagious disorder typically affecting children with a mean age of
approximately 8 years. It is rarely seen in infants and should only be
considered in the setting of patchy scaling with hair loss. Tinea capitis
usually produces scalp inflammation with mild scaling and is therefore more
easily clinically distinguished from AA.
Trichotillomania
Trichotillomania is a
psychocutaneous disorder commonly seen in children who repetitively pull out
hair from the scalp, eyebrows or eyelashes. It is classified as an impulse
control disorder with features of obsessive-compulsive disorder. Children aged
9-13 years and both sexes are commonly affected. Less commonly adults can also
be affected.
The hair loss in
trichotillomania may be asymmetrical or occur in artificial shapes. The
alopecia is not complete and short broken hairs of various lengths are usually
present across the areas of hair loss, giving a bristly texture and, unlike
exclamation mark hairs, are firmly anchored in the scalp. In most cases the
true diagnosis will become evident with time; a biopsy is useful when doubt
remains.
The pull test is
typically negative, and dermoscopy reveals question mark hairs.
There have been
reports of concomitant and isolated eyebrow and eyelash hair loss, which can
easily be mistaken for AA. In trichotillomania however the lower eyelashes are
never affected, as they are difficult to pull. In addition, patients can pull
hair from their beard, chest, underarms and pubic region. Nail biting can also
be seen with this disorder and should not be confused with other nail
abnormalities commonly seen in AA.
There have been
reports of this disorder by proxy, with parents compulsively pulling their
children's hair. Trichophagy and trichobezoars are rare disorders that can be
associated with trichotillomania and should be considered. Given all this, it
is important that psychopathology of patients and their family members be
thoroughly investigated when trichotillomania is a possibility.
Complicating matters,
AA and trichotillomania can coexist, as there has been a case reported of
concomitant trichotillomania and AA. It has been suggested that the pruritus or
other subjective symptoms can predispose children to manipulate areas of hair
loss from AA.
Scarring alopecia
Occasionally, the
early stages of scarring alopecias, including lichen planopilaris (LPP), its
clinical variant frontal fibrosing alopecia (FFA), and DLE can also mimic AA.
Dermoscopy is very helpful as it shows loss of follicular openings in addition
to the specific features of each disorder.
Prognosis and course
The
natural course of the disease is unpredictable, but often benign. In 80% of
patients with a single bald patch, spontaneous regrowth occurs within a year, but relapses at any given time are common. Alopecia
areata does not destroy hair follicles, and the potential for regrowth of hair
is retained for many years, and is possibly life-long. In a relatively small
number of patients, hair loss progresses to involve the entire scalp (alopecia
totalis) or the entire skin surface (alopecia universalis); in these cases,
spontaneous recovery is the exception rather than the rule. Patients with the "acute diffuse and total" sub-type have a favorable prognosis, regardless of treatment.
Poor
prognostic factors include:
MANAGEMENT
Treatment
studies are difficult to perform because the disease has an unpredictable
course and may improve on its own. Numerous therapies are available for the
management of alopecia areata and several can be utilized in combination, but none
has been shown to alter the course of the disease.
All
patients should be encouraged that spontaneous resolution may occur even in
longstanding and extensive alopecia areata due to the fact that hair follicles
are not destroyed. Spontaneous remission
occurs in up to 80% of patients with limited patchy hair loss of short duration
(less than 1 year). Such patients may be managed by reassurance alone, with
advice that re-growth cannot be expected within 3 months of the development of
any individual patch. The prognosis in long-standing extensive alopecia is less
favorable. However, all treatments have a high failure rate in this group and
some patients prefer not to be treated, other than wearing a wig if
appropriate.
|
TREATMENT
OPTIONS FOR THE MANAGEMENT OF ALOPECIA AREATA |
|
·
Topical and intralesional corticosteroids (1) |
|
·
Topical irritants (e.g. anthralin, tazarotene,
azelaic acid) (2) |
|
·
Topical minoxidil (2) |
|
·
Topical immunotherapy (e.g. squaric acid dibutyl
ester, diphencyprone) (1) |
|
·
Systemic corticosteroids, pulsed dosing* (especially if
rapidly progressive) (2) |
|
·
Systemic JAK/STAT pathway inhibitors: tofacitinib
(2), ruxolitinib (3) |
|
·
Topical or oral photo chemotherapy (PUVA) (2) |
|
·
Excimer laser (3) |
|
·
Systemic corticosteroids, chronic (2) |
|
·
Systemic cyclosporine (3) * E.g.
oral prednisolone 300 mg (5 mg/kg for children) monthly for a minimum of
three doses. |
To
date, topical JAK/STAT inhibitors have had a low response rate. Key to
evidence-based support: (1) prospective controlled trial; (2) retrospective
study or large case series; (3) small case series or individual case reports.
Limited hair loss
No treatment may be required, as spontaneous regrowth is common in 2-6 months.
2. Intralesional injections of corticosteroid suspensions is first line therapy for adult patients with less than 50% scalp involvement. Injections of triamcinolone, (5–10 mg/mL) are typically given either by needle injection or jet injection. Use 1ml syringe with a 30 gauze needle, steroid suspension should be placed at the level of mid dermis to target the affected miniature hair bulbs. For lesions of the scalp, 0.1 mL of triamcinolone acetonide at concentration of 5 mg/mL is injected at 1-cm intervals. Intralesional corticosteroid application is also effective for beard and eyebrow alopecia areata at a concentration of 2.5 mg/mL. Maximum dose of triamcinolone acetonide should be limited to 3 mL for scalp, 0.5 mL for each eyebrow and 1 mL for beard. Multiple injections are usually needed and repeated at intervals of 4-6 weeks as necessary. In some patients, resistance to steroid therapy can be explained by a decreased expression of thioredoxin reductase 1, an enzyme that activates the glucocorticoid receptor in the outer root sheath. Localized nonpermanent atrophy of the subcutaneous tissue is a common complication, particularly if large volumes and higher concentrations of triamcinolone are used, but this is temporary and recovers within a few months. Permanent skin atrophy can occur if the same skin area is injected repeatedly over months and years. Injection under significant pressure or with a small bore syringe increases the likelihood of retinal artery embolization. Other side effects include hypopigmentation, depigmentation, and telangiectasias. Hair regrowth is usually observed within 4 weeks in >60% of the treated patients. If no regrowth can be seen after 4 months of treatment, other treatment options should be considered. For children, intralesional injection of corticosteroids is not a suitable option. Intralesional steroid will not prevent the development of alopecia at other sites and is not suitable for patients with rapidly progressive alopecia or alopecia totalis/universalis.
3. Glucocorticoids: High potent topical steroids can be considered the mainstay therapy of this disease. They act by reducing the inflammatory infiltrate in the hair bulb and possibly promote spontaneous resolution. They are most useful for all limited form of the disease (unifocal or multifocal alopecia areata) involving the scalp. Clobetasol propionate 0.05% or betamethasone diproprionate foam/cream/solution twice daily for at least 3 months. Evidence of efficacy has been proven for class 1 corticosteroids when applied under occlusion and for class 2 corticosteroids when used in combination with minoxidil. Topical steroids should be applied 1 cm beyond the affected areas. A recent study showed that twice daily treatment with clobetasol propionate 0.05% cream used in 2 cycles of 6 weeks on, 6 weeks off regimen for a total of 24 weeks was more effective than hydrocortisone 1% cream used in the same regime. In patients suffering from alopecia totalis/universalis clobetasol propionate 0.05% under an occlusive dressing may promote further hair growth of the scalp. It can be applied overnight on six out of seven nights for six months and may promote long term hair growth. No evidence of systemic absorption was noted under this treatment in adult patients. A well known side effect of high potency topical steroids on the scalp is folliculitis. Upon extended treatment, skin atrophy will also occur.
4.
Topical irritants tretinoin 0.05% cream nightly, dithranol
cream (0.5-1%) applied overnight and azelaic acid.
5. Topical minoxidil 5%solution may be useful in limited disease (unifocal or multifocal alopecia areata) as a second line treatment. It may also be used to prevent relapse. It should be applied twice daily and may also be used for regions not suitable for topical steroid (beard and eyebrows). Better results can be achieved when minoxidil is used in combination with class II topical corticosteroids. Minoxidil shows little efficacy in alopecia totalis and universalis. Contact dermatitis and hypertrichosis are the most common side effects. Minoxidil foam, which does not contain propylene glycol, has less irritating effects than the solution.
6.
Onion juice: applied twice daily for 2 months. Mild
erythema may appear. Probably the worst smelling treatment.
Extensive hair loss
A reliable, standard therapy is not available.
2. Application of minoxidil 5%
solution or foam q.d. or b.i.d. may be attempted, but efficacy has not been
ascertained.
3.
Immunomodulator: Elemantal zinc 50mg bd for 3-6 months.
4.
Systemic glucocorticoids:
Several investigators have reported the use of pulsed oral and
intravenous corticosteroids in rapidly progressing or widespread
disease. Approximately
80% of patients will respond to high-dose systemic corticosteroids (e.g. 40 mg
triamcinolone intramuscularly monthly for 6 months or a 6‐week tapering course of oral prednisolone (starting at 40
mg/day);
however ~50% will relapse with dose reduction or cessation of therapy. However, long-term treatment is frequently needed to maintain growth,
and the attendant risks should be carefully weighed against the benefits. A 5 mg
oral dexamethasone on 2 consecutive days a week shows an excellent regrowth
after 6 months of treatment. Monthly methylprednisolone is
administered at a dose of 500 mg/day for 3 days or 5 mg/kg twice a day for 3
days in children. More than 60% of patients with widespread patchy alopecia
responded. Half of the patients with alopecia totalis had a good response,
while a quarter of those with universal alopecia responded. Patients with
ophiasic alopecia areata did not respond. Systemic corticosteroid should not be
used as routine treatments because they do not alter the long-term
prognosis and can cause side effects such as striae, acne, obesity, cataracts
and hypertension.
5.
Induction of
Allergic Contact Dermatitis–Topical
immunotherapy (with diphencyprone or squaric acid dibutylester) is a
non-FDA-approved treatment for extensive disease. Contact immunotherapy
is the most effective and best-documented treatment for corticosteroid
refractory, prolonged and extensive disease (alopecia areata totalis and
universalis), but is available in only a few centers. The patient is sensitized
to a potent allergen and the same allergen is then applied to the scalp,
usually at weekly intervals, in a concentration sufficient to induce a mild
contact dermatitis. The contact allergens that have been used in the treatment
of alopecia areata include diphenylcyclopropenone (DPCP) and squaric acid
dibutylester (SADBE). Most centers now use DPCP. 50–60% of patients achieve a
worthwhile response. Patients with extensive hair loss are less likely to
respond. Other reported adverse prognostic features include the presence of
nail changes, early onset and a positive family history. Treatment is
discontinued after 6 months if no response was obtained. The response in
patients with alopecia totalis and universalis is less favorable. Most
patients will develop occipital and/or cervical lymphadenopathy during contact
immunotherapy. This is usually temporary but may persist throughout the
treatment period. Severe dermatitis is the most common adverse event, but the risk
can be minimized by careful titration of the concentration. Cosmetically
disabling pigmentary complications, both hyper- and hypo pigmentation
(including vitiligo), may occur if contact immunotherapy is used in patients
with racially pigmented skin.
The
mode of action of contact immunotherapy is unknown. Happle suggested that the
contact allergen competes for CD4 cells, attracting them away from the
perifollicular region (‘antigenic competition’). Other suggested mechanisms include
non-specific stimulation of a local T-suppressor-cell response and increased
expression of TGF-β in the skin, which acts to suppress the immune response. Almost all patients respond and many stay free of disease for
long intervals, although recurrence are the rule.
|
TOPICAL IMMUNOTHERAPY FOR ALOPECIA
AREATA |
|
Treatment protocol for diphencyprone (DPCP) or squaric acid dibutyl
ester (SADBE)* |
|
· Sensitize with a 2%
solution in acetone applied to a 4 × 4 cm area on one side of the scalp · After 1 week (2 weeks
if the initial reaction is severe), a 0.001% solution is applied to all
affected areas on the same side of the scalp · Each week, DPCP or
SADBE is applied to the same side of the scalp >The concentration is titrated according to the
severity of the reaction the previous week, with a goal of maintaining a
low-grade, tolerable degree of erythema, scaling, and pruritus for 24–36
hours after application >Concentrations are increased
incrementally as follows: 0.001%, 0.01%, 0.025%, 0.05%, 0.1%, 0.25%, 0.5%,
1%, 2% · Once hair growth is
established, the other side is also treated · Initial responses are
usually seen after 12 weeks, and therapy is discontinued if there is no
response by 24 weeks · Frequency of treatment
can be decreased in the presence of full regrowth |
|
Additional information |
|
· Acetone solutions
should be applied using a generous amount of cotton at the end of a wooden
stick† · Following application: >The sensitizer is
left on the scalp for 48 hours, then washed off >Patients should avoid
touching the scalp for 6 hours >The scalp should be protected from light to avoid
degradation of DPCP · SADBE must be
refrigerated · Side effects include
lymphadenopathy, severe or widespread eczematous reactions, and post
inflammatory pigment alterations (particularly in patients with darkly
pigmented skin) |
|
Contraindications |
|
· Pregnancy (although
teratogenicity has not been established) ·
Malignancies and blood dyscrasias * Both not FDA-approved. † Application by medical personnel is
recommended. |
Immunosuppressive treatment
Immunosuppressive agents namely sulfasalazine, methotrexate, and cyclosporine can be used in the treatment of alopecia areata.
Sulfasalazine therapy can be an alternative treatment option in persistent alopecia areata cases. Studies have shown favorable treatment response, but a high relapse rate. The most common side effects include nausea, vomiting, headache, fever, and rash; less commonly hematologic abnormalities and hepatotoxicity can develop.
Severe forms of alopecia areata resistant to conventional topical and/or systemic treatments may respond to methotrexate. In a retrospective study, weekly 15–25 mg methotrexate with or without 10–20 mg prednisolone daily was reported to be effective in 64% of cases.
Cyclosporine has been used alone or in conjunction with corticosteroids variable response rates. Use of cyclosporine is limited because of side effects and high relapse rate. Side effects include nephrotoxicity, immune suppression, hypertension, and hypertrichosis of body hair.
JAK inhibitors
Recent data support
the role of Janus kinase (JAK) mediated pathways in alopecia areata. Interferon
gamma, interleukin-2 and IL-15 play a significant role in maintaining the auto
reactive CD8+T cell infiltrate in AA. Their receptors signal through JAK1, JAK2
and JAK3. The inhibition of these cytokine receptors with JAK inhibitors can
lead to a reversal of AA.
Tofacitinib
citrate is a small-molecule selective Janus kinase 1/3 (JAK 1/3) inhibitor that
was approved by FDA, in late 2012, for the treatment of moderate-to-severe
rheumatoid arthritis. A patient
with longstanding alopecia universalis, treated with tofacitinib for psoriasis
had hair regrowth, being the first documented case of alopecia areata
responding to tofacitinib. After eight months of tofacitinib treatment
(5 mg twice daily for 2 months followed by 10 mg in the morning and
5 mg at night thereafter), the patient had full regrowth of hair at all
body
sites. There have been other case
reports showing efficacy of tofacitinib treatment. Adverse effects of
tofacitinib use include increased risk of severe infections including
tuberculosis, anemia, neutropenia, headache, and mild nausea.
Another
JAK inhibitor, ruxolitinib applied topically twice daily for 12 weeks in a
patient with refractory alopecia universalis induced almost full eyebrow
regrowth and approximately 10% regrowth of scalp hair.
Several case reports
show that JAK inhibitors are promising class of drugs for AA even in cases of
severe or widespread disease. Oral baricitinib and tofacitinib citrate have also
shown a good treatment outcome with full regrowth of scalp hair in widespread
AA.
Summary
Alopecia areata is
difficult to treat. The tendency to spontaneous remission and the lack of
adverse effects on general health are important considerations in management,
and counselling, with no treatment, is the best option in many cases. Topical
and intralesional corticosteroids can be helpful in disease of limited extent.
Topical minoxidil is widely used but there is little convincing evidence of
efficacy. Contact immunotherapy is the most effective treatment for extensive
alopecia areata, although it is not widely available, and the response rate in
alopecia totalis and universalis is low. The place of systemic corticosteroids
is controversial.
Alopecia areata may
cause considerable psychological and social disability. If the prognosis is
poor (e.g. in a prepubertal atopic child with total alopecia), a full
explanation and help in adjusting to the problems of hair loss will be of far
greater value than the raising of unwarranted hopes.
What else should be
considered for alopecia areata?
Counselling
Some
people with alopecia areata seek and benefit from professional counselling to
come to terms with the disorder and regain self-confidence.
Camouflaging hair loss
Scalp
A
hairpiece is often the best solution to disguise the presence of hair loss.
These cover the whole scalp or only a portion of the scalp, using human or
synthetic fibres tied or woven to a fabric base.
A full wig is a cap that fits over the whole head.
A partial wig must be clipped or glued to existing hair.
A hair integration system is a custom-made hair net that
provides artificial hair where required, normal hair being pulled through the
net.
Hair
additions are fibres glued to existing hair and removed after 8 weeks
Styling
products include gels, mousses and sprays to keep hair in place and add volume.
They are reapplied after washing or styling the hair.
Eyelashes
Artificial
eyelashes come as singlets, demilashes and complete sets. They can be trimmed
if necessary. The lashes can irritate the eye and eyelids. They are stuck on
with methacrylate glue, which can also irritate and sometimes causes contact
allergic dermatitis.
Eyeliner
tattooing is permanent and should be undertaken by a professional cosmetic
tattooist. The colour eventually fades and may move slightly from the original
site. It is extremely difficult to remove the pigment, should the result turn
out to be unsatisfactory.
Eyebrows
Artificial
eyebrows are manufactured from synthetic or natural human hair on a net that is
glued in place.
Eyebrow
pencil can be obtained in a variety of colours made from inorganic pigments.
Tattooing
can also be undertaken to disguise the loss of eyebrows, but tends to look
rather unnatural because of the shine of hairless skin.