Pemphigus
Salient features
1.
Pemphigus
is a group of autoimmune blistering diseases of the skin and mucous membranes
that is characterized by:
o
histologically,
intra epidermal blisters due to the loss of cell–cell adhesion of keratinocytes
o
immunopathologically,
the finding of in vivo bound and circulating
IgG auto antibodies directed against the cell surface of keratinocytes
2.
Pemphigus
is divided into three major forms: pemphigus vulgaris, pemphigus foliaceus, and
paraneoplastic pemphigus
3.
The
functional inhibition of desmogleins, which play an important role in cell–cell
adhesion of keratinocytes, by IgG auto antibodies results in blister formation
4.
Patients
with pemphigus vulgaris and pemphigus foliaceus have IgG auto antibodies
against desmoglein 3 and desmoglein 1, respectively, while patients with
paraneoplastic pemphigus also have IgG auto antibodies against plakin molecules
as well as a T-cell-mediated autoimmune reaction that leads to an interface
dermatitis
5.
IgA
pemphigus is characterized by IgA, but not IgG, auto antibodies directed
against keratinocyte cell surfaces and is divided into two major subtypes:
intra epidermal neutrophilic (IEN) type and sub corneal pustular dermatosis
(SPD) type
6.
Systemic
corticosteroids are a mainstay of therapy in pemphigus vulgaris, given the
rapidity of clinical response, but because of their potential side effects at
effective doses, they are combined with steroid-sparing agents
7.
These
additional therapies include immunosuppressive medications such as
mycophenolate mofetil, high-dose IVIg (non-immunosuppressive), and rituximab;
in the future, the latter may become a first-line therapy
|
|
|
|
|
|
|
|
Abstract
Pemphigus is a group of IgG
autoantibody-mediated blistering diseases of the skin and mucous membranes that
includes three major forms: pemphigus vulgaris, pemphigus foliaceus, and
paraneoplastic pemphigus. Histologically, there is intra epidermal blister formation
due to the loss of cell–cell adhesion of keratinocytes. Immunopathologic
studies serve to identify in vivo bound
and circulating IgG auto antibodies against desmogleins found within
desmosomes. Patients with pemphigus vulgaris and pemphigus foliaceus have IgG auto
antibodies against desmoglein 3 and desmoglein 1, respectively, while patients
with paraneoplastic pemphigus also have IgG auto antibodies against plakin
molecules as well as a T-cell-mediated autoimmune reaction that leads to
interface dermatitis. Systemic corticosteroids are a mainstay of therapy, but
due to their toxicity at effective doses, immunosuppressive medications are
regularly used as steroid-sparing agents. More recently, high-dose IVIg, which
is non-immunosuppressive, and rituximab, a B-cell-depleting monoclonal
antibody, have been added to the therapeutic armamentarium for pemphigus.
Introduction
Pemphigus
is a group of chronic blistering skin diseases in which autoantibodies are
directed against the cell surface of keratinocytes, resulting in the loss of
cell–cell adhesion of keratinocytes through a process called acantholysis.
Pemphigus can be divided into three major forms: pemphigus vulgaris, pemphigus
foliaceus, and paraneoplastic pemphigus.
Pemphigus vulgaris and pemphigus foliaceus are the classic
forms of pemphigus. All patients with pemphigus vulgaris have mucosal membrane
erosions, and more than half will also have cutaneous blisters and erosions.
The blisters of pemphigus vulgaris develop in the deeper portion of the
epidermis, just above the basal cell layer. Patients with pemphigus foliaceus
have only cutaneous involvement without mucosal lesions, and the splits occur
in the superficial part of the epidermis, mostly at the granular layer. Pemphigus
vegetans is a variant of pemphigus vulgaris, and pemphigus erythematosus
represent a localized variant of pemphigus foliaceus.
More recently, paraneoplastic pemphigus is recognized as a
disease distinct from the classic forms of pemphigus, as it
consists of both humoral and cellular autoimmune reactions. Patients with paraneoplastic pemphigus have a known or
occult neoplasm, usually of lymphoid tissue. Painful, severe oral and
conjunctival erosions are a prominent feature of paraneoplastic pemphigus.
IgA pemphigus is characterized by IgA, but not IgG, auto
antibodies directed against keratinocyte cell surfaces and is divided into two
major subtypes:
|
|
• |
Intra
epidermal neutrophilic (IEN) type, with pustule formation throughout the
entire epidermis |
|
|
• |
Sub corneal pustular dermatosis (SPD) type, with pustules
primarily in the upper epidermis. |
|
CLASSIFICATION OF PEMPHIGUS |
|||||||||||||||||||||||||||
|
Target
antigens in pemphigus A2ML1, alpha-2-macroglobulin-like-1 protease inhibitor;
BPAG1, bullous pemphigoid antigen 1
|
TARGET ANTIGENS IN
PEMPHIGUS |
|||
|
Disease |
Autoantibodies |
Antigens |
MW (kDa) |
|
Pemphigus vulgaris |
|||
|
Mucosal-dominant type |
IgG |
Desmoglein 3 |
130 |
|
Mucocutaneous type |
IgG |
Desmoglein 3 |
130 |
|
Desmoglein 1 |
160 |
||
|
Pemphigus foliaceus |
IgG |
Desmoglein 1 |
160 |
|
Paraneoplastic pemphigus |
IgG |
Desmoglein 3 |
130 |
|
Desmoglein 1 |
160 |
||
|
Plectin* |
500 |
||
|
Epiplakin* |
500 |
||
|
Desmoplakin I* |
250 |
||
|
Desmoplakin II* |
210 |
||
|
BPAG1* |
230 |
||
|
Envoplakin* |
210 |
||
|
Periplakin* |
190 |
||
|
A2ML1 |
170 |
||
|
Drug-induced pemphigus |
IgG |
Desmoglein 3 |
130 |
|
Desmoglein 1 |
160 |
||
|
IgA pemphigus† |
|||
|
Subcorneal pustular dermatosis
type |
IgA |
Desmocollin 1 |
110/100 |
|
Intraepidermal neutrophilic
type |
IgA |
? |
? |
* Members of plakin family.
† A subset of patients has IgA
autoantibodies against Dsg1 or Dsg3.
Epidemiology
Incidence and
prevalence
The incidence of pemphigus is low. Pemphigus vulgaris
(PV) is generally the commoner form, though there is some geographical
variation in the incidence of the different subtypes; thus PV is more common in
Europe, the US and India whereas pemphigus foliaceus (PF) is more common in
Brazil and Africa.
Age
PV can occur at any age but is usually seen between the
fourth and sixth decades of life. In India, patients with PV have a relatively
low age of onset of disease (mean 40 years).
Sex
Pemphigus seems to affect men and women equally.
Ethnicity
PV has been reported in all ethnic groups, but is more
common in Indian populations. Genetic variations are likely to play a major
role.
The
intraepidermal immunobullous diseases: characteristic clinical features
The
intraepidermal immunobullous diseases: immunopathology and immunogenetics
Pathophysiology
Pathogenic
Autoantibodies in Pemphigus
The hallmark of pemphigus is the finding of IgG auto
antibodies against the cell surface of keratinocytes. The pemphigus
autoantibodies found in patients’ sera are pathogenic because they induces the
loss of cell adhesions between keratinocytes, and subsequent blister formation.
Neonates of mothers with pemphigus vulgaris may have a transient
disease caused by maternal IgG that crosses the placenta. As maternal antibody
is catabolized, the disease subsides.
Desmogleins as
Pemphigus Antigens
Immunoelectron microscopy localized pemphigus vulgaris and
pemphigus foliaceus antigens to the desmosomes, the most prominent cell–cell
adhesion junctions in stratified squamous epithelia. The basic
pathophysiology of pemphigus is as follows: autoantibodies inhibit the adhesive
function of desmogleins and lead to the loss of the cell–cell adhesion of
keratinocytes, resulting in blister formation.
Compelling
evidence has accumulated that IgG auto antibodies against Dsg1 and Dsg3 are
pathogenic and play a primary role in inducing the blister formation in
pemphigus. Essentially, all patients with pemphigus have IgG auto antibodies
against Dsg1 and/or Dsg3, depending on the subtype of pemphigus. When
anti-desmoglein IgG auto antibodies are removed from the sera of patients with
pemphigus vulgaris, pemphigus foliaceus or paraneoplastic pemphigus (by
immunoadsorption with recombinant desmoglein proteins), the sera are no longer
pathogenic in inducing blister formation.
Desmoglein
Compensation Theory as Explanation for Localization of Blisters
The sites of blisters in pemphigus vulgaris and foliaceus
are explained logically by the desmoglein compensation theory: Dsg1 and Dsg3
compensate for each other when they are co expressed in the same cell. The principal target
antigens in pemphigus are desmogleins (Dsg) 1 and 3, which are expressed in the
skin and mucosal tissue. However, the distribution of the two proteins varies
in different epithelia, such that in skin, Dsg 1 is
expressed throughout
the epidermis, but more intensely in the
superficial layers whereas
Dsg 3 is found only in the parabasal and immediate suprabasal layers. In oral
epithelium, both Dsg1 and Dsg3 are expressed through all layers
but Dsg 1 is only present at a much lower
level than Dsg3.
While patients with pemphigus foliaceus have only anti-Dsg1
IgG auto antibodies, individuals with the mucosal-dominant type of pemphigus
vulgaris have only anti-Dsg3 IgG auto antibodies. Those with the mucocutaneous
type of pemphigus vulgaris have both anti-Dsg3 and anti-Dsg1 IgG auto
antibodies.
Logical explanation for the localization of blister formation in
classic pemphigus by desmoglein compensation theory
The colored triangles represent the distribution of
desmoglein 1 (Dsg1) and desmoglein 3 (Dsg3) in the skin (A) and mucous
membranes (B). Pemphigus foliaceus sera contain only anti-Dsg1 IgG, which
causes superficial blisters in the skin because Dsg3 functionally compensates
for the impaired Dsg1 in the lower part of the epidermis (A1), whereas those
antibodies do not cause blisters in the mucous membranes because cell–cell
adhesion is mainly mediated by Dsg3 (B1). Sera containing only anti-Dsg3 IgG
cause no or only limited blisters in the skin because Dsg1 compensate for
the loss of Dsg3 mediated adhesion (A2); however, these sera induce separation
in the mucous membranes, where the low expression of Dsg1 will not compensate
for the loss of Dsg3-mediated adhesion (B2). When sera contain both anti-Dsg1
and anti-Dsg3 IgG, the function of both Dsgs is compromised and blisters occur
in both the skin and mucous membranes (A3, B3). In neonatal skin, the situation
is similar to that shown here for mucous membranes.
When sera contain only anti-Dsg1 IgG (which interferes with
the function of Dsg1), blisters appear only in the superficial epidermis of the
skin because that is the only area in which Dsg1 is present without co
expression of Dsg3. In the unaffected deep epidermis, the presence of Dsg3
compensates for the loss of function of Dsg1. Although the anti-Dsg1 IgG binds
to mucosa, no blisters are formed, because of the co expression of Dsg3. Thus, sera
containing only anti-Dsg1 IgG cause superficial blisters in the skin without
mucosal involvement, as are seen in patients with pemphigus foliaceus.
When sera contain only anti-Dsg3 IgG, they are inefficient
in producing cutaneous blisters because co expressed Dsg1 compensates for the
impaired function of Dsg3, resulting in no, skin lesions. However, in the
mucous membranes, Dsg1 cannot compensate for the impaired Dsg3 function because
of its low expression. Therefore, a serum containing only anti-Dsg3 IgG cause
oral erosions without apparent skin involvement, as is seen in patients with
the mucosal-dominant type of pemphigus vulgaris.
When sera contain both anti-Dsg1 and anti-Dsg3 IgG, they
interfere with the function of both Dsg1 and Dsg3, resulting in extensive
blisters and erosions of the skin as well as the mucous membranes, as is seen
in patients with the mucocutaneous type of pemphigus vulgaris. It is not clear why
splits appear just above the basal layer instead of the whole epithelium
falling apart. However, it is speculated that cell–cell adhesion in the
suprabasal and parabasal layers might be weaker than in other parts of the
epithelium because there are fewer desmosomes. In addition, autoantibodies,
which penetrate from the dermis, might have better access to the lower part of
the epithelia.
In pregnant women with pemphigus, autoantibodies cross the
placenta and bind to the fetal epidermis. However, neonates develop blisters if
the mother has pemphigus vulgaris, but very rarely if she has pemphigus
foliaceus. This confusing observation is also explained by the desmoglein
compensation theory. The distribution of Dsg3 within neonatal epidermis is
unlike that in adult epidermis; it is found on the surface of keratinocytes
throughout the epidermis, which is similar to its distribution in mucous
membrane. Therefore, pemphigus foliaceus sera containing only anti-Dsg1 IgG
cannot induce blisters in neonatal skin.
In pemphigus,
the disruption of cell–cell adhesion is currently thought to be mediated via
the combined effects of direct inhibition by antibodies plus subsequent signal
transduction induced by antibody binding. The direct inhibition is mediated by
steric hindrance, i.e. the binding of auto antibodies to desmogleins spatially
interferes with the adhesive interaction of desmogleins between cells.
Humoral and Cellular Autoimmunity in Paraneoplastic
Pemphigus
Patients
with paraneoplastic pemphigus develop characteristic IgG auto antibodies
against multiple antigens, including Dsg3 and/or Dsg1, multiple members of the
plakin family (plectin, epiplakin, desmoplakins I and II, bullous pemphigoid
antigen 1, envoplakin, and periplakin), and the protease inhibitor alpha-2-macroglobulin-like-1.
Anti-desmoglein antibodies play a role in inducing the loss of cell adhesion of
keratinocytes and initiate blister formation, while the pathophysiologic
relevance of the anti-plakin auto antibodies is unclear, in that plakin molecules
are intracellular and IgG cannot penetrate cell membranes. In addition to
humoral autoimmunity, cell-mediated cytotoxicity is involved in the
pathogenesis of paraneoplastic pemphigus, in which more severe and refractory
oral erosions and stomatitis as well as more polymorphic skin eruptions are
seen, in comparison with classic forms of pemphigus. It is demonstrated that
Dsg3-specific CD4+ T cells not only help B cells produce anti-Dsg3 IgG (which
causes acantholysis), but also directly infiltrate into the epidermis and
induce an interface dermatitis. Clarification of the exact roles of autoimmune
T cells should provide valuable insights into the pathophysiology of
paraneoplastic pemphigus.
The direct and indirect immunofluorescent (IF) staining pattern
of paraneoplastic pemphigus differs from that of classic forms of pemphigus. In
perilesional skin, direct IF shows deposition of IgG and the third component of
complement (C3) on epidermal cell surfaces as well as variably along the
basement membrane zone. Unlike classic forms of pemphigus, in which
autoantibodies only bind to stratified squamous epithelia, as detected by
indirect IF, auto antibodies in paraneoplastic pemphigus also react with simple
or transitional epithelia such as urinary bladder epithelium. The latter can be
used to differentiate paraneoplastic pemphigus from classic pemphigus.
Immunologic Mechanism of Pathogenic Autoantibody Production
in Pemphigus
In contrast to the significant
progress in understanding the pathophysiologic mechanisms of blister formation
in pemphigus, it is still unclear why patients with pemphigus begin to produce
the pathogenic autoantibodies.
Pemphigus
autoantibodies are composed of IgG isotypes, which may be produced after
isotype switching, and they have a high affinity towards the antigen, which may
be a result of affinity maturation of the antibodies. In addition, pemphigus
sera recognize several distinct epitopes on desmogleins, and the presence of
autoantibodies is associated with specific HLA class II alleles, including
DRB1*0402, DRB1*1401 and DQB1*0302 in Caucasians and DRB1*14 and DQB1*0503
in Japanese. All of these features suggest that autoantibody production in
pemphigus is T cell-dependent. More recently, T cells reactive against Dsg3 are
shown to be present in peripheral blood from patients with pemphigus vulgaris
as well as healthy individuals. Certain peptides from Dsg3, predicted to fit
into the DRB1*0402 pocket, are able to stimulate T cells from the pemphigus
patients.
Acantholysis
The key pathological process in PV is separation of
keratinocytes from one another, a change known as acantholysis. Mechanisms
include steric hindrance by anti‐Dsg antibodies.
Environmental factors
A number of reports have suggested that smoking may have
a protective or beneficial role in pemphigus. Human keratinocytes have both
nicotinic and muscarinic receptors for acetylcholine and these receptors may
play a role in regulating keratinocyte cell–cell adhesion.
Pesticides have also been postulated as possible triggers
in disease development and an increased risk of pemphigus has been shown in
exposed individuals. Organophosphate pesticides block the acetylcholine
breakdown pathway and so may lead to acetylcholine accumulation with resulting
loss of cell–cell adhesion in the epidermis.
A link between diet and disease development in pemphigus
has been suggested but difficult to prove. Although garlic has been proposed as
a trigger for disease development – and has been shown to induce acantholysis
in vitro – this area remains controversial.
Drug‐induced pemphigus
Drug‐induced pemphigus is rare. Approximately 80% of cases are due to drugs that contain a
thiol group, such as penicillamine, ACE inhibitors (e.g. captopril), gold
sodium thiomalate, and pyritinol. Non-thiol drugs include antibiotics
(especially β-lactams), nifedipine,
phenobarbital, piroxicam, propranolol, and pyrazolone derivatives. Up to 10% of cases of pemphigus may be
drug-induced. Lesions characteristic of pemphigus foliaceus or pemphigus
vulgaris appear several weeks or months after the responsible drug is begun.
Penicillamine is the most common culprit in drug‐induced
pemphigus and the disease may occur in 3–10% of patients on the drug, typically
after around 1 year of exposure. Penicillamine‐induced
pemphigus tends to occur in individuals with other autoimmune disorders such as
rheumatoid arthritis suggesting that immune dysregulation may be an underlying
factor. Genetic factors may also play a role as an increase in frequency of HLA‐B15
has been reported in penicillamine‐induced pemphigus. In
some patients, simple withdrawal of the drug is sufficient to induce remission
though in others treatment with corticosteroids and immunosuppressive
medication may be required.
Clinical features
Pemphigus vulgaris
Essentially
all PV patients usually start with
painful erosions in the oral mucosa and remain
localized for months, or
may be the only manifestation of the disease, or extend to involve the skin
(average lag period of 4 months), after
which generalized bullae may occur. Less frequently there may be a generalized,
acute eruption of bullae from the beginning.
Pemphigus vulgaris is therefore divided into two subgroups: (1) the mucosal-dominant
type with mucosal erosions but minimal skin involvement; and (2) the mucocutaneous
type with extensive skin blisters and erosions in addition to mucosal
involvement.
Mucous membrane lesions usually present as painful erosions.
Intact blisters are rare, probably because they are fragile and break easily.
Although erosions may be seen anywhere in the oral cavity, the most common
sites are the buccal and palatal mucosa. The erosions are of different sizes
with an irregular ill-defined border and are slow to heal, which, when extensive or painful, may result in decreased oral intake of
food or liquids. The erosions extend peripherally with shedding of the
epithelium. The diagnosis of pemphigus
vulgaris tends to be delayed in patients presenting with only oral involvement,
as compared to patients with skin lesions.
The lesions may extend out onto the
vermilion lip and lead to thick, fissured hemorrhagic crusts. Involvement of
the throat produces hoarseness and difficulty in swallowing. The esophagus also
may be involved with sloughing of its entire lining in the form of a cast. Other mucosal
surfaces may be involved, including the conjunctiva, nasal mucosa, larynx, urethra, penis,
anus, vulva
and vagina. Cytology of vaginal cells may be misread
as a malignancy when vaginal lesions are present.
Most patients develop cutaneous
lesions. Involvement occasionally remains localized to one site but more
commonly becomes widespread.
The
primary skin lesions of pemphigus vulgaris are flaccid, thin-walled, easily
ruptured blisters. They can appear anywhere on the skin surface and arise on
either normal-appearing skin or erythematous bases. Common sites of predilection are scalp, face, neck, upper chest, axillae, groin, umbilicus and
back. The fluid within the bullae is
initially clear but may become hemorrhagic, turbid, or even seropurulent. As
the blisters are fragile they soon rupture to form painful erosions that ooze
and bleed easily. These erosions extend at the edges as more epidermis is
lost which often attain a large size and can
become generalized. The erosions soon become partially covered with crusts that
have little or no tendency to heal. Those lesions that do heal often leave hyper
pigmented patches with no scarring. Associated pruritus is uncommon.
Because
of an absence of cohesion within the epidermis, firm sliding pressure with a finger
will separate normal‐looking
epidermis from dermis, producing erosion in
patients with active disease (Nikolsky sign). The lack of cohesion of the skin
may also be demonstrated with the “bulla-spread phenomenon” – gentle pressure
on an intact bulla forces the fluid to spread under the skin away from the site
of pressure (Asboe–Hansen sign, also referred to as the “indirect Nikolsky” or
“Nikolsky II” sign). Without appropriate treatment, pemphigus vulgaris can be
fatal because a large area of the skin loses its epidermal barrier function,
leading to the loss of body fluids or to secondary bacterial infections
Lesions in skin folds may form vegetating granulations,
and flexural PV merges with its variant pemphigus vegetans. Nail dystrophies,
acute paronychia and subungual hematomas have been observed in pemphigus.
Additionally, some patients will undergo phenotypic and immunological
conversion from PV to PF or vice versa over the course of their disease.
Pemphigus may deteriorate in pregnancy and the puerperium.
In some patients, initial presentation
is in pregnancy. Severe pemphigus in pregnancy may be associated with fetal
prematurity and death. Generally, the baby is healthy although neonatal
pemphigus may occur with mucosal or mucocutaneous lesions which are generally
short lived.
Pemphigus vegetans
Pemphigus
vegetans is a rare vegetative variant of pemphigus vulgaris,
characterized by vegetating erosions, and
it is thought to represent a granulomatous
reactive pattern of the skin to the
autoimmune insult of pemphigus vulgaris. Lesions are seen primarily
in the flexures and on the scalp or face.
Two subtypes are recognized: the severe Neumann type and the mild Hallopeau
type.
Patients have circulating antibodies against Dsg 3, as in PV. In some cases,
antibodies in patients with pemphigus vegetans react with desmocollin
molecules.
In
neumann type: vesicles and bullae rupture to form erosions and then form granulomatous vegetating plaques.
In
hallopeau type: Pustules rather than vesicles characterize early lesions, but
these soon progress to vegetative plaques.
The disease chiefly affects middle‐aged adults. Involvement
of the oral mucosa is almost invariable, often with cerebriform changes on the
tongue.
A
vegetative response may occasionally also be seen in lesions of PV that tends
to be resistant to therapy and remain localized for long period of time in one
location.
Pemphigus foliaceus
Patients
with pemphigus foliaceus develop well‐demarcated scaly, crusted
cutaneous erosions, often on an erythematous base, sometimes with small
vesicles along the borders, but they do not have
clinically apparent mucosal involvement even with widespread disease.
The onset
of disease is often subtle, with a few scattered crusted lesions that are
transient and are frequently mistaken for impetigo. They have a seborrheic distribution, i.e. they favor the
face, scalp and upper trunk (chest and upper back).
Because the vesicle is so superficial and fragile, often only the resultant
crust and scale are seen, the scales have been likened to cornflakes. The
disease may stay localized for years or it may rapidly progress, in some cases
to generalized involvement and become erythrodermic with crusted oozing red skin. The Nikolsky sign is present. In contrast to the extensive
oral lesions in pemphigus vulgaris, it is extremely rare, if ever, for patients
with pemphigus foliaceus to develop mucosal involvement. Generally, patients
with pemphigus foliaceus are not severely ill. They do complain of burning and
pain in association with the skin lesions.
Although the antibodies in PF can cross the placenta, the
neonate is not usually affected.
Pemphigus erythematosus
Pemphigus
erythematosus is simply a localized variant of pemphigus foliaceus, originally
described by Senear and Usher.
Typical erythematous scaly and crusted lesions of
pemphigus foliaceus appear on the nose and malar region of the face in
a butterfly distribution
and in other “seborrheic” areas. Sunlight may exacerbate the
disease. Originally, the term “pemphigus erythematosus”
was introduced to describe patients with immunologic features of both lupus
erythematosus and pemphigus, i.e. in vivo IgG and C3 deposition on cell
surfaces of keratinocytes as well as the basement membrane zone, in addition to
circulating antinuclear antibodies. However, only a few patients have been
reported to actually have the two diseases concurrently. The
antibodies recognize Dsgs together with Ro, La and double‐stranded
DNA antigens. Progression to systemic lupus erythematosus is rare. Pemphigus
erythematosus may be associated with myasthenia gravis or thymoma.
Herpetiform Pemphigus
Most
patients with herpetiform pemphigus have a clinical variant of pemphigus
foliaceus and the remainder may have a variant of pemphigus vulgaris. This
disorder is characterized by: (1) erythematous urticarial plaques and tense
vesicles that present in a herpetiform arrangement; (2) eosinophilic spongiosis
and sub corneal pustules with minimal or no apparent acantholysis
histologically; and (3) IgG auto antibodies directed against the cell surfaces
of keratinocytes. The target antigen is Dsg1 in most cases and Dsg3 in the
remainder. Some patients with herpetiform pemphigus will have features of
pemphigus foliaceus or vulgaris during the course of their disease, and some
patients will evolve into having pemphigus foliaceus or vulgaris. It is assumed
that the pathogenic blister-inducing activity of the IgG auto antibodies in
herpetiform pemphigus might be weaker than that seen in classic forms of
pemphigus. Although often clinically less severe than pemphigus vulgaris, the
course may be more chronic.
Paraneoplastic pemphigus
Paraneoplastic pemphigus is associated with underlying
neoplasms, both malignant and benign. The most commonly associated neoplasms
are non-Hodgkin lymphoma (40%), chronic lymphocytic leukemia (30%), Castleman's
disease (10%), malignant and benign thymomas (6%), sarcomas (6%) and
Waldenström's macroglobulinemia (6%). Non-Hodgkin lymphoma and chronic
lymphocytic leukemia together account for two-thirds of patients.
The most constant clinical feature of paraneoplastic
pemphigus is the presence of intractable stomatitis. Severe stomatitis is
usually the earliest presenting sign and, after treatment, it is the one that
persists and is extremely resistant to therapy. This stomatitis consists of
erosions and ulcerations that affect all surfaces of the oropharynx and
characteristically extend onto the vermilion lip. Most patients also have a
severe pseudomembranous conjunctivitis, which may progress to scarring and
obliteration of the conjunctival fornices. Esophageal, nasopharyngeal, vaginal,
labial and penile mucosal lesions may also be seen.
Cutaneous findings are quite
polymorphic and may present as erythematous macules, flaccid blisters and
erosions resembling pemphigus vulgaris, tense blisters resembling bullous
pemphigoid, erythema multiforme-like lesions, and lichenoid eruptions. The
occurrence of blisters and erythema multiforme-like lesions on the palms and
soles is often used to differentiate paraneoplastic pemphigus from pemphigus
vulgaris, in which lesions on the palms and soles are unusual. In the chronic
form of the disease, a lichenoid eruption may predominate over blistering
lesions. Some patients with paraneoplastic pemphigus develop bronchiolitis
obliterans, which can be fatal as a result of respiratory failure. Although
its pathophysiologic mechanism is still unclear, ectopic expression of
epidermal antigens in the setting of squamous metaplasia is thought to render
the lung a target organ. Of note, a chest X-ray or CT scan obtained at
the onset of bronchiolitis obliterans may be normal but pulmonary function
tests will show small airway obstruction that does not reverse with
bronchodilators.
IF changes are characteristic, with features of both
pemphigus and pemphigoid reflecting the broad spectrum of circulating
antiepithelial antibodies.
|
DISORDERS WITH HEMORRHAGIC
CRUSTS OF THE VERMILION LIPS |
|
1.
Herpes
simplex 2.
Herpes
zoster 3.
Erythema
multiforme major 4.
Stevens–Johnson
syndrome/TEN spectrum 5.
Pemphigus
vulgaris 6.
Paraneoplastic
pemphigus 7.
Contact
cheilitis |
IgA pemphigus
IgA pemphigus
represents a more recently characterized group of autoimmune intra epidermal
blistering diseases presenting with a vesiculopustular eruption, neutrophilic
infiltration of the skin, and in vivo bound and circulating IgA
autoantibodies against the cell surface of keratinocytes, but with no IgG auto
antibodies. IgA pemphigus usually occurs in middle-aged or elderly persons. Two
distinct types of IgA pemphigus have been described: the sub corneal pustular dermatosis
type and the intra epidermal neutrophilic type.
Patients with both types of IgA pemphigus present with
flaccid vesicles or pustules on either erythematous or normal skin. In both
types, the pustules tend to coalesce to form an annular or circinate pattern
with crusts in the center of the lesion with accumulation of the pustular
component in the dependent portion of the vesiculopustule seen in (SPD) type,
and a sunflower-like configuration of pustules is a characteristic sign of the
intra epidermal neutrophilic type. The most common sites of involvement are the
axilla and groin, but the trunk, face, scalp and
proximal extremities can also be involved. Mucous membrane involvement is rare,
and pruritus is often a significant symptom. Because the sub corneal pustular dermatosis
type of IgA pemphigus is clinically and histologically indistinguishable from
classic sub corneal pustular dermatosis (Sneddon–Wilkinson disease),
immunologic evaluation is essential to differentiate the two diseases.
IgA
deposition on cell surfaces of epidermal keratinocytes is present in all cases,
as shown by direct immunofluorescence (DIF) microscopy, and many patients have
detectable circulating IgA autoantibodies, as shown by indirect
immunofluorescence (IIF) microscopy. In the sub corneal pustular dermatosis
type, IgA auto antibodies tend to react against upper epidermal surfaces, while
in the intra epidermal neutrophilic type, IgA auto antibodies are found
throughout the entire epidermis. The subclass of IgA autoantibodies is
exclusively IgA1. IgA auto antibodies in the sub corneal pustular dermatosis
type are shown to recognize desmocollin 1, while the autoimmune targets of the
intra epidermal neutrophilic type remain to be identified. A subset of IgA
pemphigus patients have IgA autoantibodies directed against Dsg1 or Dsg3,
making the autoimmune targets of IgA pemphigus more heterogeneous. The exact
pathogenic role of IgA auto antibodies in inducing pustular formation in IgA
pemphigus remains to be elucidated.
|
|
Drug-Induced Pemphigus
There are sporadic cases of pemphigus associated with the
use of drugs, in particular penicillamine and captopril. In patients receiving
penicillamine, pemphigus foliaceus is seen more commonly than pemphigus
vulgaris, with a ratio of approximately 4: 1. Although most patients with
drug-induced pemphigus are shown to have autoantibodies against the same
molecules involved in sporadic pemphigus, some drugs may induce acantholysis
without the production of antibodies. Both penicillamine and captopril contain sulfhydryl
groups that interact with the sulfhydryl groups in Dsg1 and Dsg3. This
interaction may modify the antigenicity of the desmogleins, which may lead to
autoantibody production, or their interaction may directly interfere with the
adhesive function of the desmogleins. Most, but not all, patients with
drug-induced pemphigus go into remission after the offending drug is
discontinued.
The
clinical, histologic, and immunofluorescent microscopy findings in drug-induced
pemphigus are similar to those in the spontaneous form of the disease, except
that direct immunofluorescence of perilesional skin is positive in only 90% of
cases. Circulating anti-desmoglein auto antibodies are found in ~70% of
patients.
Spontaneous
remission after drug withdrawal is not always observed, especially in those
patients in whom the reaction is due to drugs that do not contain a thiol
moiety.
Investigations
Histopathology
It is essential to take a biopsy
from an early lesion in order to establish the correct diagnosis because
pemphigus blisters rupture easily.
If
the lesion is small enough, the entire vesicle can be removed for routine
histology. If the lesions are not small, the edge of a fresh vesicle or bulla
plus the inflammatory rim is recommended. Examination
of perilesional, rather than lesional, skin is recommended for DIF in order to
avoid negative staining due to secondary degeneration of target antigens and
immunoreactants. In patients with only mucosal lesions, the biopsy specimen
should consist of the active border of a denuded area, since intact blisters
are rarely encountered. Cytologic examination (Tzanck smear) is useful for the
rapid demonstration of acantholytic epidermal cells within the blister cavity.
However, this bedside test merely represents a preliminary diagnostic tool and
it should not supplant histologic examination. This is because acantholytic
keratinocytes are occasionally seen in various non-acantholytic vesiculobullous
or pustular diseases as a result of secondary acantholysis.
Preferred sites for obtaining biopsy specimens in autoimmune bullous
diseases
If
the lesion is small enough, the entire vesicle can be removed for routine
histology. If the lesions are not small, the edge of a fresh vesicle or bulla
plus the inflammatory rim is recommended. For direct immunofluorescence (DIF)
for various forms of pemphigus and bullous pemphigoid, perilesional skin is
preferred, whereas nearby normal skin is recommended in dermatitis
herpetiformis.
Pemphigus Vulgaris
The characteristic histologic finding in this form of
pemphigus is intra epidermal blister formation due to a loss of cell–cell
adhesion of keratinocytes (acantholysis) without keratinocyte necrosis. Whereas
acantholysis usually occurs just above the basal cell layer (supra basilar acantholysis),
intraepithelial separation may occasionally occur higher in the stratum
spinosum. A few rounded-up (acantholytic)
keratinocytes as well as clusters of epidermal cells and a few inflammatory
cells, notably eosinophils, are often seen in the blister cavity. Although the
basal cells lose lateral desmosomal contact with their neighbors, they maintain
their attachment to the basement membrane via hemidesmosomes, thus giving the
appearance of a “row of tombstones”. The border of a blister on the buccal
mucosa shows intraepithelial separation in the lower part of the mucosal
epithelia. The acantholytic process may involve hair follicles.
The dermal papillary outline is usually maintained and,
frequently, the papillae protrude into the blister cavity. The blister cavity
may contain a few inflammatory cells, notably eosinophils, and in the dermis
there is a moderate perivascular mononuclear cell infiltrate with conspicuous
eosinophils. In rare instances, the earliest histologic finding consists of
eosinophilic spongiosis, in which eosinophils invade a spongiotic epidermis
with little or no evidence of acantholysis.
In pemphigus vegetans, supra basilar acantholysis is seen,
in addition to considerable papillomatosis and acanthosis. Characteristically,
there is an intense inflammatory cell infiltrate containing numerous
eosinophils, and intra epidermal micro abscesses are often seen.
Pemphigus Foliaceus
The histologic changes of pemphigus foliaceus and pemphigus
erythematosus are identical. Early blisters in pemphigus foliaceus have
acantholysis in the upper epidermis, within or adjacent to the granular layer.
As the blisters are superficial and fragile, it is often difficult to obtain an
intact lesion for histologic examination. As a result, acantholysis is
sometimes difficult to detect, but usually a few acantholytic keratinocytes can
be found attached to the roof or floor of the blister. Sometimes the blister
cavity contains numerous acute inflammatory cells, particularly neutrophils.
Eosinophilics pongiosis can be also seen in very early lesions of pemphigus
foliaceus. The dermis shows a moderate number of inflammatory cells, among
which eosinophils are often present.
|
|
Paraneoplastic
Pemphigus
The histologic findings of cutaneous lesions in
paraneoplastic pemphigus show considerable variability, reflecting the
polymorphism seen clinically. The lesions show a unique combination of pemphigus
vulgaris-like, erythema multiforme-like, and lichen planus-like histologic
features, sometimes in the same specimen. Intact cutaneous blisters demonstrate
supra basilar acantholysis and individual keratinocyte necrosis with
lymphocytes within the epidermis. In addition, basal cell liquefactive
degeneration or a band-like dense lymphocytic infiltrate in the upper dermis
can be seen. Eosinophils are rare. Biopsy specimens of the severe ulcerative
stomatitis usually yield only nonspecific changes of inflammation, but the
perilesional oral epithelium should show supra basilar acantholysis.
IgA Pemphigus
The characteristic histologic feature in IgA pemphigus is
formation of an intra epidermal pustule or vesicle. The contents of the
pustules consist predominantly of neutrophils. Acantholysis is usually not
seen. IgA pemphigus is divided into two subtypes depending on the level of
intra epidermal pustule; in the sub corneal pustular dermatosis type, pustules
are located subcorneally in the upper epidermis, while in the intra epidermal neutrophilic
type, pustules involving the lower (supra basal) or entire epidermis are
present.
A. DIF is performed on skin biopsy specimens in order to
detect in vivo bound IgG. B. IIF is
performed utilizing patients' sera in order to detect circulating
autoantibodies that bind epithelial antigens.
Direct
immunofluorescence detects antibodies in a patient’s
skin. Here immunoglobulin G (IgG) antibodies are detected by staining with a
fluorescent dye attached to antihuman IgG.
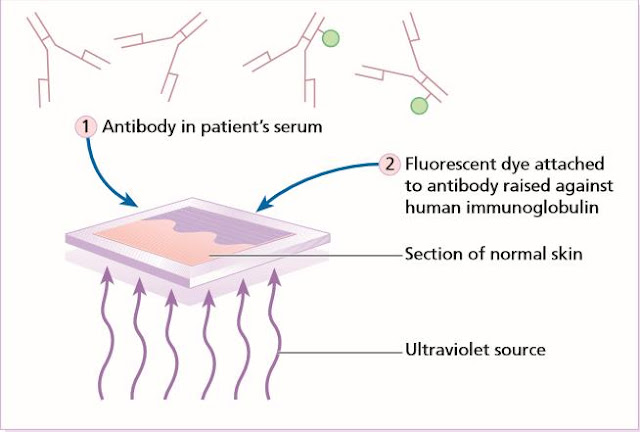
Indirect
immunofluorescence detects antibodies in a patient’s
serum. There are two steps. (1) Antibodies in this serum are made to bind to
antigens in a section of normal skin. (2) Antibody raised against human
immunoglobulin, conjugated with a fluorescent dye can then be used to stain
these bound antibodies (as in the direct immunofluorescence test).
Indirect immunofluorescence (IIF) for
pemphigus and bullous pemphigoid – recommended substrates
|
INDIRECT
IMMUNOFLUORESCENCE (IIF) – RECOMMENDED SUBSTRATES |
|
|
Type of pemphigus |
Recommended substrates
(autoantibodies) |
|
Pemphigus vulgaris |
Monkey esophagus (anti-Dsg3) |
|
Pemphigus foliaceus |
Human skin or guinea pig esophagus
(anti-Dsg1) |
|
Paraneoplastic pemphigus |
Monkey and guinea pig esophagus
(anti-Dsg1, anti-Dsg3) |
|
Bullous pemphigoid |
Human skin, salt-split |
|
Mucous membrane (cicatricial)
pemphigoid |
Human skin, salt-split; normal
oral or genital mucosa or conjunctiva |
Diagnosis
For BP180, the NC16A domain is utilized and for BP230, the
N- and/or C-terminus. BP, bullous pemphigoid; EBA, epidermolysis bullosa acquisita;
MMP, mucous membrane pemphigoid; PF, pemphigus foliaceus; PNP, paraneoplastic
pemphigus; PV, pemphigus vulgaris.
Immunofluorescence (red) in bullous diseases
Disease course and
prognosis
Before
the advent of glucocorticoid therapy, PV was almost invariably fatal; most patients died within 2–5 years of the onset of the
disease because large areas of the skin lost their epidermal barrier function,
leading to the loss of body fluids or to secondary bacterial infections.
Pemphigus foliaceus had a better prognosis, except for the occasional acute
cases with generalized involvement.
The
systemic administration of glucocorticoids and the use of immunosuppressive
therapy have dramatically improved the prognosis for patients with pemphigus;
however, pemphigus is still a disease associated with a significant morbidity
and mortality. Infection is often the cause of death, and by causing the
immunosuppression necessary in the treatment of active disease, therapy is
frequently a contributing factor. With glucocorticoid and immunosuppressive
therapy, the mortality (from disease or therapy) of PV patients is
approximately 10% or less, whereas that of PF is probably even less.
Pemphigus in its
various forms typically has a chronic course with average disease duration of
10 years. Various factors have been suggested to influence this including the
site and severity of initial disease, with oral involvement an adverse
prognostic factor. Immunologically, the presence of both Dsg 1 and 3 antibodies
tends to associate with more active disease. Recent data suggest that early age
of onset and Asian ancestry associate with more prolonged disease activity. With the advent of rituximab therapy, complete remission in pemphigus may
become more common.
Management
Pemphigus Vulgaris
General
principles of management
PV
is an uncommon and potentially life-threatening disease requiring
immunosuppressive treatment. The management of active oral PV with systemic
therapies should be approached in the same way as the management of active skin
disease. The management of PV can be considered in two main phases: induction
of remission and maintenance of remission.
Remission
induction
In
remission induction the initial aim of treatment is to induce disease control,
defined as new lesions ceasing to form and established lesions beginning to
heal. Corticosteroids are the most effective and rapidly acting treatment for PV;
hence they are critical in this phase. Using corticosteroids, disease control
typically takes several weeks to achieve (median 3 weeks). During this phase
the intensity of treatment may need to be built up rapidly to suppress disease
activity. Although adjuvant drugs are often initiated during this phase, their
immediate therapeutic benefit is relatively limited because of their slower
onset. They are rarely used alone to induce remission in PV. After disease
control is achieved there follows a consolidation phase during which the drug
doses used to induce disease control are continued. The end of this
consolidation phase is defined arbitrarily as being reached when 80% of lesions
have healed, both mucosal and skin, and there have been no new lesions for at
least 2 weeks. This phase may be relatively short, but could be considerably
longer if there is extensive cutaneous erosion. Healing of oral ulceration
tends to take longer than that for skin, with the oral cavity often the last
site to clear in those with mucocutaneous PV. The end of the consolidation
phase is the point at which most clinicians would begin to taper treatment,
usually the corticosteroid dose. Premature tapering of corticosteroids, before
disease control is established and consolidated, is not recommended.
Remission
maintenance
After
induction there follows maintenance phase during which treatment is gradually
reduced, in order to minimize side-effects, to the minimum required for disease
control. The ultimate goal of treatment should be to maintain remission on
prednisolone 10 mg daily or less, with 10 mg being the dose designated
arbitrarily as ‘minimal therapy’ by international consensus. PV is a chronic
disease, and in one study 36% of patients required at least 10 years of
treatment. Systemic corticosteroids are the most important element of remission
induction and consolidation. In general, adjuvant drugs are slower in onset
than corticosteroids. Their main role is in remission maintenance. Adjuvant
drugs are combined commonly with corticosteroids with the aim of increasing
efficacy and reducing maintenance corticosteroid doses and subsequent
corticosteroid side-effects.
British Association of Dermatologists’ guidelines for
the management of pemphigus vulgaris 2017
An
overview of PV management
First-line therapy
Corticosteroids
•
Oral prednisolone – optimal dose not established but suggest start with
prednisolone 1 mg kg per day (or equivalent) in most cases, 0.5–1 mg kg in
milder cases
•
Increase in 50–100% increments every 5–7 days if blistering continues
•
Consider pulsed intravenous corticosteroids if > 1 mg kg oral prednisolone
required, or as initial treatment in severe disease followed by 1 mg kg per day
oral prednisolone
•
Taper dose once remission is induced and maintained, with absence of new
blisters and healing of the majority of lesions (skin and mucosal). Aim to
reduce to 10 mg daily or less
•
Assess risk of osteoporosis immediately
•
Effective in all stages of disease, including remission induction
Combine
corticosteroids with an adjuvant immunosuppressant
•
Azathioprine 2–3 mg kg per day (if TPMT normal)
•
Mycophenolate mofetil 2–3 g per day
•
Rituximab (rheumatoid arthritis protocol, 2 x 1 g infusions, 2 weeks apart)
•
More important for remission maintenance than induction, due to delayed onset
Good
skin and oral care are essential
Second-line therapy
·
Consider switching to alternate
corticosteroid-sparing agent if treatment failure with first-line adjuvant drug
(azathioprine, mycophenolate mofetil or rituximab) or mycophenolic acid
720–1080 mg twice daily if gastrointestinal symptoms from mycophenolate mofetil
Third-line therapy
·
Consider choice of additional treatment
options based on assessment of individual patient need and consensus of
multidisciplinary team.
Options include
•
Cyclophosphamide
•
Immunoadsorption
•
Intravenous immunoglobulin
•
Methotrexate
•
Plasmapheresis or plasma exchange
TPMT,
thiopurine methyl transferase. Rituximab
is currently approved by National Health Service England as a third-line
treatment for pemphigus. Treatment
failure is defined by international consensus as continued disease activity or
failure to heal despite 3 weeks of prednisolone 1.5 mg kg per day, or
equivalent, or any of the following, given for 12 weeks: (i) azathioprine 2.5
mg kg per day (assuming normal TPMT), (ii) mycophenolate mofetil 1.5 g twice
daily, (iii) cyclophosphamide 2 mg kg per day, (iv) methotrexate 20 mg per
week.
PV is a serious disease, so even if the disease is
limited in extent at the onset should be treated aggressively with systemic
corticosteroids combine with immunosuppressive, because it will ultimately
generalize and the prognosis without therapy is very poor.
Because PV is caused by
pathogenic autoantibody and
there is relationship between pemphigus autoantibody and the disease activity,
therapy is aimed at both resolution of cutaneous lesions and elimination of
circulating antibody, not just to suppress local inflammation. The introduction of systemic corticosteroids and
immunosuppressive agents has greatly improved the prognosis of pemphigus;
however, the morbidity, and occasional mortality, is still significant due to
complications of therapy. Systemic corticosteroids are the mainstay of therapy
for pemphigus. Immunosuppressive agents are often used for their
corticosteroid-sparing effect in order to reduce the side effects of the
corticosteroids, with the goal of therapy being to control the disease with the
lowest possible dose of corticosteroids. The titer of the circulating antiepithelial
antibody should be determined at the onset of treatment. The life-threatening
nature of pemphigus mandates aggressive therapy. The earlier
therapy is initiated, the greater the likelihood of success. Patients usually require many
months of immunosuppressive therapy, making systemic steroid monotherapy
inappropriate. Instead, early in the treatment course, begin a steroid-sparing
agent. More recently, high-dose IVIg, which is non-immunosuppressive, and
rituximab have been added to the therapeutic armamentarium.
Monitoring activity of disease
In the acute phase the progress of the
disease should be evaluated clinically. Once blistering stops and erosions
heal, changes in the titer of circulating pemphigus antibody (IIF test titers and ELISA values) may
help in guiding the dose of steroids. It should be monitored about every 6 months, and
should decrease with each measurement. Direct
immunofluorescence studies of normal skin have also been recommended to predict
remission or relapse. Laboratory monitoring is essential for
hematologic and metabolic indicators of glucocorticoid and/ or
immunosuppressive-induced adverse effects.
Topical
therapy
Patients who present with oral disease
and mild cutaneous involvement may remain in this localized phase for months.
Potent topical steroid such as clobetasol propionate 0.05% cream or
intralesional steroids may reduce the requirement for oral steroids. Topical
anticholinergic gel (pilocarpine gel) is reported to help healing of oral
erosions. Good oral hygiene, including treatment of periodontal disease, is
important.
Opportunist infection is the major
cause of death in patients with widespread blistering who are also
immunosuppressed. Potassium permanganate and topical antiseptics may help
reduce the risk of cutaneous infection, whilst topical imidazoles will reduce the
risk of oral candida.
Corticosteroids and immunosuppressive agents
Systemic corticosteroid therapy,
usually in the form of oral prednisone, is the mainstay of therapy. Prednisone at 1 mg/kg/day (usually 60 mg/day) is a
typical initial dosage. Very-high-dose regimens (more than 120 mg/day)
provide no benefit over the low-dose regimens with respect to the frequency of
relapse or the incidence of complications.
The majority of
pemphigus vulgaris patients present with oral disease at an early and relatively
stable stage. The following regimen, also known as Lever's mini treatment
(LMT), is used. These patients may be controlled by starting prednisone 40 mg
on alternate days plus 100 mg azathioprine every day until there is complete
healing of all lesions. A gradual monthly and later bimonthly decrease of
prednisone was followed by the tapering of azathioprine, in a 1-year period.
The time required for the epithelialization of lesions varies between 4
and 7 months.
The combination
therapy of mycophenolate mofetil (MMF) and prednisone is reported to be an
effective treatment regimen to achieve rapid and complete control of
PV. For those patients who fail treatment with MMF and prednisone,
rituximab is an efficacious alternative therapy. Complete disease control is
achieved in 90% of patients using the following treatment algorithm.
If complete
remission is achieved with the combined therapy, the dosage of the
immunosuppressive drug is maintained while the prednisone is slowly tapered;
when a dose of 5–10 mg/day is reached, careful tapering of the
immunosuppressive drug is attempted. In young patients, the potential increase
in malignancies that might be associated with the use of these drugs must be
taken into account.
The dosage of prednisone is tapered to a level that controls
most disease activity. Attempts are made to use an alternate-day regimen to
minimize side effects. One taper method is to reduce prednisone by 10 mg every
week until the daily dose reaches 20 mg. Then the dose is reduced each
alternate week until a dose of 20 mg on alternate days is reached. Then the
dose reduction is slower until a final dose of 5 mg on alternate days is
achieved.
During the prednisone taper, the
immunosuppressive agent is continued at full dosage. The speed of the
prednisone taper is determined by the level of disease activity. It is not
necessary to have the disease totally suppressed before lowering the prednisone
dose.
In some
patients, especially those who are elderly with limited disease or those in
whom corticosteroids are contraindicated, immunosuppressive agents alone may be
used. Patients who fail to respond to
corticosteroids and immunosuppressive agents can be treated with rituximab or
intravenous immunoglobulin (IVIG).
Immunosuppressive
drugs
Immunosuppressive
agents are given concomitantly for their glucocorticoid-sparing effect;
systemic steroids are routinely combined with other immunosuppressive drugs with
the expectation that other agent leads to a reduced total steroid dose,
increased remission rate and fewer side effects. These side effects include
infection, DM, osteoporosis, aseptic bone necrosis, thrombosis, cataract and
peptic ulcers.
The most commonly used agents are
mycophenolate mofetil, azathioprine and cyclophosphamide. One study showed that
mycophenolate mofetil and azathioprine had similar efficacy,
corticosteroid-sparing-effects, and safety profiles as adjuvants during
treatment of pemphigus vulgaris and pemphigus foliaceus.
Mycophenolate mofetil
Mycophenolate mofetil
(1 g twice daily) has been reported to
be beneficial. It has a similar action to azathioprine, with less
myelosuppression but more gastrointestinal toxicity.
Adverse effects are gastrointestinal disorders (most common), genitourinary
complaints, increased incidence of viral or bacterial infection, and neurologic
symptoms. Relative contraindications include lactation, peptic ulcer disease,
hepatic or renal disease, and concomitant azathioprine or cholestyramine
therapy.
Azathioprine
Azathioprine, most frequently used agent. In the past, it was given in the
dose of 1.5- 2.5 mg/kg body weight. Today, the dose is adjusted based on
individual activity level for thiopurinemethyl transferase (TPMT), which should
be determined prior to initiating therapy. The onset of action of AZA is 4-6
weeks. The dose is continued at this level until the systemic steroids are
completely tapered and stopped. After 1-2 month of monotherapy, the dose is AZA
is tapered. If no new lesion occur and the circulating antibodies are no longer
detected, oral mucosal biopsy is taken once a dose of 50mg/day has been
reached. If the biopsy is negative, one can safely assume that the disease is
truly in remission and withdraw the agent. Azathioprine causes bone marrow suppression,
hepatotoxicity, and an increased risk of malignancy that is lower than that of
cyclophosphamide. Monitor blood cell counts and liver function tests.
Cyclophosphamide
Average starting dosage is 100 mg (1.1
to 2.5 mg/kg) per day. Cyclophosphamide “bolus” therapy with
500 mg IV given on day 1 of DCP along with mesna; during the interval 50mg
daily for the first 6 months is given.
Cyclophosphamide may be the most effective
drug but it is toxic. Side effects include bone marrow suppression, sterility, hemorrhagic
cystitis, bladder fibrosis, reversible alopecia, and an increased risk of
bladder carcinoma and lymphoma. Monitor urinalysis and blood cell counts.
Encourage oral fluid intake to decrease the risk of bladder fibrosis and
hemorrhagic cystitis.
Rituximab
Rituximab is a
potent B-cell-depleting chimeric anti-CD20 monoclonal antibody, presumably targets B cells, the
precursors of (auto) antibody-producing plasma cells. CD20 is a transmembrane glycoprotein specifically expressed on B
cells (from the pre-B stage in the bone marrow to the activated and memory B
stage in blood or secondary lymphoid organs), but its expression is lost upon
plasma cell differentiation.
Rituximab seems not only to induce a depletion of CD20+ B cells and
a decline in IgG (including anti-desmoglein auto antibodies), but also
decreases desmoglein-specific T cells. When used as
adjuvant therapy, rituximab led to a complete remission in most of the patients
with refractory pemphigus vulgaris and foliaceus. Two infusions of 1
g given on day 1 and day 15.
For maintenance: 500mg at 12 months and every 6 months thereafter to maintain
clinical remission or 1gm dose if clinical relapse occurs. Onset of action of rituximab is typically 8–16
weeks following the first infusion and improvement may persist for 12–18
months. The combination of rituximab and intravenous immunoglobulin is
effective in patients with refractory pemphigus vulgaris.
Adverse effects of rituximab in the treatment of
pemphigus appear uncommon. Because rituximab is a
chimeric biologic agent, patients may develop anti-drug antibodies that are
associated with infusion reactions and a lower therapeutic response.
Infection has been reported, and particular care needs to be taken to avoid
reactivation of viral hepatitis – patients should be rigorously screened prior
to treatment.
Veltuzumab, a humanized anti-CD20 antibody
which may be administered intravenously or subcutaneously, is reported to be an
effective treatment in an individual patient.
In the future,
desmoglein-specific immune suppression, via targeting T cells or B cells, needs
to be developed. Recently, for example, the possibility of using modified CAR
(chimeric antigen receptor) therapy to target Dsg3-specific B cells was
proposed. Such approaches would represent an ideal therapeutic strategy given
that the target antigens and pathophysiological mechanisms of pemphigus have
been well characterized.
Intravenous immunoglobulin
High-dose IVIg is
another option for resistant disease. IVIg is a blood product prepared from
pooled plasma that has immunomodulatory effects when used in a high dose. It is
thought to exert its effect via multiple modes of action, including modulation
of expression and function of Fc receptors and the cytokine network; provision
of anti-idiotypic antibodies; and modulation of dendritic cell, T-cell and
B-cell activation, differentiation and effector functions. High-dose intravenous
immunoglobulin (400
mg/kg/day for 5 consecutive days) in a single cycle is an effective and safe
treatment for patients with pemphigus who are relatively resistant to systemic
steroids.
Management of blisters
1.
Gently cleanse blister with antimicrobial
solution, taking care not to rupture
2.
Pierce blister at base with a sterile needle,
with the bevel facing up. Select a site in which the fluid will drain out by
gravity to discourage refilling
3.
Gently apply pressure with sterile gauze
swabs to facilitate drainage and absorb fluid
4.
Do not de-roof the blister
5.
After fluid has drained, gently cleanse again
with an antimicrobial solution
6.
It may be necessary to apply a non adherent
dressing
7.
Some large blisters may need a larger hole to
drain properly – use a larger needle and pierce more than once
8.
Many patients report pain or a burning
sensation during blister care; offer analgesia prior to the start of the
procedure
9.
Document on blister chart the number and
location of new blisters
Course and remission
It is possible to eventually induce complete
and durable remissions in most patients with pemphigus that permit systemic
therapy to be safely discontinued without a flare in disease activity. The
proportion of patients in whom this can be achieved increases steadily with
time, and therapy can be discontinued in approximately 75% of patients after 10
years.
Determining remission and when
to stop treatment
Treatment is stopped when patients are
clinically free of disease and when they have a negative finding on direct
immunofluorescence. The titers of circulating antibodies have a rough
correlation with disease activity, but they are not accurate enough to
determine when to stop therapy. A skin biopsy for direct immunofluorescence can
predict when a patient is in remission and may be used to predict relapse. A
negative direct immunofluorescence finding suggests that there is immunologic
remission, and 80% of patients with a negative direct immunofluorescence study
remained disease free for the next 5 years.
Pemphigus Foliaceus
When pemphigus foliaceus is active and widespread, the
treatment is, in general, similar to that for pemphigus vulgaris. In some
patients, pemphigus foliaceus may be localized for many years; they do not
necessarily have to be treated with systemic therapy, and super potent topical
corticosteroids may be sufficient to control the disease. The combination of
nicotinamide (1.5 gm/day) and minocycline (100 mg twice a day) may be an
effective alternative to steroids in pemphigus foliaceus. Dapsone can also be used when neutrophils are dominant
histologically.
Paraneoplastic Pemphigus
Patients with benign tumors, such as thymomas or localized
Castleman's disease, should have the tumor surgically excised. The majority of
these patients will either improve substantially or clear completely. However,
it may take 6–18 months to see complete resolution of lesions after excision of
a benign neoplasm. In patients with malignant neoplasms, there is no consensus
on a standard effective therapeutic regimen. Administration of tumor-specific
chemotherapy may result in complete resolution of the malignancy and a slow
resolution of the skin lesions. Cutaneous lesions respond more rapidly to
therapy, in contrast to the stomatitis, which is generally refractory to most
forms of treatment. Overall, the prognosis of paraneoplastic pemphigus is poor
due to its resistant nature to treatment.
IgA Pemphigus
Dapsone is the drug of choice for most patients with IgA
pemphigus. A clinical response usually occurs within 24–48 hours. If dapsone is
not well tolerated, sulfapyridine and acitretin are useful alternatives.
Occasionally, those drugs are not effective, and low- to medium-dose prednisone
may be considered, as well as photo chemotherapy (PUVA) or colchicine.