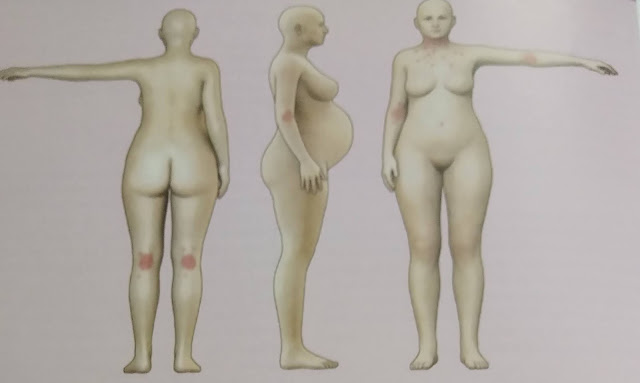
Pregnancy dermatoses
Introduction
The dermatoses of pregnancy
are all associated with severe pruritus. The diagnoses of pemphigoid gestationis
and intrahepatic cholestasis of pregnancy are confirmed by immunofluorescence
and laboratory findings respectfully.
Polymorphic and atopic eruptions of pregnancy are distressing only to
the mother. Pemphigoid gestationis may be associated with prematurity and
small-for-gestational age babies, and intrahepatic cholestasis of pregnancy
increases the risk for fetal distress, premature labour, and stillbirth.
Corticosteroids and antihistamines control pemphigoid gestationis as well as
polymorphic and atopic eruptions of pregnancy. Intrahepatic cholestasis of
pregnancy is treated with ursodeoxycholic acid.
Summary of
Dermatoses of Pregnancy
|
Morphology |
Distribution |
Usual Onset |
Fetal Risk |
|
|
|
Pemphigoid
(herpes) gestationis |
Urticarial
papules and plaques progress to vesicles and bullae |
Begins on trunk,
then progresses to generalized eruption Spares face,
mucus membranes, palms, soles |
Second or third
trimester, or immediately postpartum |
Small-for-gestational
age births Preterm delivery Neonatal
pemphigoid gestationis |
|
|
Intrahepatic
cholestasis of pregnancy |
Excoriations and
excoriated papules ± jaundice |
Localized to
palms and soles or generalized |
Third trimester |
Preterm delivery Fetal distress Fetal death |
|
|
Pustular
psoriasis of pregnancy |
Erythematous
patches with subcorneal pustules at their margins |
Begins in
flexures Generalizes
demonstrating centrifugal spread |
Third trimester |
Placental
insufficiency may lead to stillbirth or neonatal death |
|
|
Pruritic
urticarial papules and plaques of pregnancy |
“Polymorphous”
including urticarial papules and plaques ± vesicles |
Begins within
abdominal striae Spreads to
remainder of trunk and then extremities Spares umbilicus |
Third trimester
or immediately postpartum |
None |
|
|
E-type Atopic
eruption of pregnancy |
Eczematous
patches and plaques |
Face, neck,
chest, flexural extremities |
Second or third
(less commonly) trimester |
None |
|
|
P-type Atopic
eruption of pregnancy |
Excoriated or
crusted papules |
Extremities,
occasionally trunk |
Second or third
(less commonly) trimester |
None |
NA = not applicable.
|
DERMATOSES OF PREGNANCY – FETAL RISK, INVOLVEMENT OF
NEWBORN SKIN, AND RISK OF RECURRENCE |
|||
|
Dermatosis |
Fetal risk |
Newborn skin
involvement |
Risk of recurrence |
|
Pemphigoid gestationis |
Increased risk of
prematurity and small-for-gestational age neonates; risk correlates with
disease severity |
Mild and transient
lesions of pemphigoid gestationis in up to 10% |
Commonly recurs
(“skipped” pregnancies in only 5–8% of women); recurrences induced by oral
contraceptives in 25–50% |
|
Polymorphic eruption
of pregnancy |
None |
None |
Usually does not recur |
|
Intrahepatic
cholestasis of pregnancy |
Increased risk of
premature labor (20–60%), intrapartal fetal distress (20–30%), and
stillbirths (1–2%) |
None |
Recurrence in 45–70%
of subsequent pregnancies; may be triggered by oral contraceptives |
|
Atopic eruption of
pregnancy |
None |
None |
Commonly recurs due to
atopic diathesis |
Dermatoses Not Associated with Fetal Risk in Pregnancy
At a Glance
|
·
Pruritic urticarial papules and plaques of
pregnancy is a common, self-limited, intensely pruritic dermatosis that
occurs almost exclusively in primigravidas during late pregnancy. The term polymorphic
eruption of pregnancy appropriately encompasses the wide spectrum of
clinical presentations. ·
Atopic eruption of pregnancy represents a newly
introduced complex comprising pruritic folliculitis of pregnancy, prurigo of
pregnancy, and eczema of pregnancy. Lesions typically appear before the third
trimester and may resemble classic atopic dermatitis (AEP, E-type) or be
papular (AEP, P-type). |
Polymorphic
eruption of pregnancy
Salient
features
·
Urticarial papules and plaques that usually first appear within
striae distensae during the latter portion of the third trimester or
immediately postpartum
·
Development of polymorphous features (vesicles, erythema,
target, and eczematous lesions) with disease progression
·
Most frequent in primiparous women
·
Nonspecific histologic features, negative IF, and normal routine
laboratory evaluation
·
No maternal or fetal risks; usually does not recur
Introduction
Polymorphic
eruption of pregnancy is a common, benign, self‐limiting,
intensely pruritic inflammatory disorder that occurs almost exclusively in primigravidae
(76%) and begins late in the third trimester of
pregnancy (mean onset, 35 weeks) or occasionally in the early postpartum period.
It is characterized by a typical
clinical presentation, normal laboratory tests, and negative IF or ELISA.
Epidemiology
Its
incidence is about 1: 160 pregnancies. It is seen predominantly in
primiparous women and tends not to recur in subsequent pregnancies. There is
neither an autoimmune diathesis nor an association with a specific HLA type.
Pathophysiology
The pathogenesis of PEP remains unclear. The main theories
proposed focus on abdominal distension and hormonal and immunological factors. It has
therefore been suggested that rapid, late stretching of abdominal skin may lead
to damage of connective tissue with altered collagen and/or elastic fibers and
elicitation of an allergic-type reaction, resulting in the initial appearance
of the eruption within striae. The lesions then become generalized as the
inflammatory response develops cross-reactivity to collagen in otherwise
normal-appearing skin. Immune tolerance during subsequent pregnancies might
prevent recurrence. Increased
progesterone receptor immunoreactivity has been detected in
lesional PUPPP, leading some to posit a role for progesterone activation of
keratinocytes.
Clinical
features
Typical distribution of PEP
Onset is most
often during the latter part of the third trimester (85%), usually 1 to 2 weeks before delivery or in
the immediate postpartum period (15%). The earliest lesions are severely pruritic 1- to 2-mm, erythematous, urticarial papules
surrounded by a narrow pale halo. The papules coalesce to form erythematous edematous urticarial
plaques with polycyclic shape and arrangement. The eruption appears suddenly,
begins on the abdomen in 90% of patients, classically within the striae gravidarum, and
demonstrates periumbilical sparing. Skin lesions are extremely pruritic, causing
sleepless nights and great stress, yet excoriations are infrequent. After a few days, rapid spread of eruption in a symmetric
fashion to the proximal
thighs, buttocks, lower
back, breasts,
and upper
inner arms is the
norm. Involvement of the palms, soles, or skin above the breast is exceptional.
There
are no mucous membrane lesions. While pruritic
urticarial papules are the initial lesions in almost all patients,
approximately half will develop more polymorphic features as the disease
evolves, including widespread non‐urticated erythema, erythema
multiforme–like target lesions, tiny vesicles,
(1–2 mm in size; never bullae), and
eczematous plaques. Irrespective of whether the eruption starts during
pregnancy or postpartum, lesions resolve over an average of 6 weeks, independently of delivery.
Disease
course and fetal prognosis
Maternal
and fetal prognosis is unimpaired and there is no cutaneous involvement of the
newborn. Lesions are self‐limiting and recurrences in subsequent pregnancies or with
exposure to oral contraceptives are unusual. Spontaneous remission within days
of delivery is the rule.
Differential Diagnosis
Since lesions of PEP may show
microvesiculation, contact dermatitis must be considered. Drug eruptions,
urticaria, or viral exanthems may also be in the clinical differential
diagnosis. The most important entity to exclude is urticarial pemphigoid gestationis,
whose lesions tend to appear earlier during gestation, have no association with
abdominal striae and often involve the umbilicus, along with positive IF of
perilesional skin.
Diagnosis
The diagnosis is generally made
clinically when patient presents with the eruption in typical locations at the
end of pregnancy. Biopsy should be performed if PG is being considered in the
differential diagnosis. The histopathology of this
condition is non‐specific. Epidermal changes vary from modest
spongiosis to acanthosis with hyperkeratosis and parakeratosis, depending upon
the stage of the disease. The dermis shows a nonspecific perivascular
lymphocytic infiltrate with a variable degree of dermal edema and a variable
number of neutrophils or eosinophils. Early lesions may resemble arthropod bite
reactions,
with a superficial and deep perivascular and
interstitial lymphohistiocytic infiltrate within the dermis. Numerous
eosinophils are also present and an absence of epidermal changes.
The histologic correlate of microvesiculation is
severe epidermal spongiosis and/or dermal edema.
Direct
and Indirect immunofluorescence is generally negative.
Treatment
Although harmless to the mother and fetus, pruritus is often intense and
unremitting. Symptomatic treatment with topical
corticosteroids and emollients, with or without antihistamines, is usually
sufficient to control pruritus and skin lesions. In severe generalized cases, a
short course of systemic corticosteroids (prednisolone starting at 40–60 mg/day
and tapering to zero over a few weeks)
relieves
symptoms in 24 h. Early induction of labor can also be
considered if the patient is close to term.
Treatment ladder
First line
·
Topical emollients: aqueous cream +
1–2% menthol
·
Topical corticosteroids
·
Oral antihistamines: loratadine and
cetirizine
Second line
·
Prednisolone
Consider early induction of labor if patient is close to term
Atopic eruption of
pregnancy (AEP)
Salient
features
·
Eczematous and/or papular skin lesions in a patient with an
atopic diathesis in whom other specific dermatoses have been excluded
·
Most common pruritic disorder during pregnancy
·
Generally appears earlier than other pregnancy-related
dermatoses (75% before the third trimester)
·
Nonspecific histology; negative direct IF; elevated serum IgE
levels in up to 70% of patients
·
No maternal or fetal risks; commonly recurs in subsequent
pregnancies
Introduction
Atopic
eruption of pregnancy (AEP) is defined as a
benign pruritic disorder of pregnancy characterized by either
an exacerbation or the first occurrence of eczematous (AEP, E-type) and/or papular
(AEP, P-type) skin changes during pregnancy in patients
with an atopic diathesis. As the majority of patients belong to the
second group, the atopic
link is often overlooked, leading to a number of different diagnoses, as
evidenced by the many synonyms.
AEP comprises about 50% of all
pregnancy dermatoses.
It
usually starts before the third trimester, and a tendency to recur in
subsequent pregnancies.
Epidemiology
AEP is by far the
most common pruritic disorder in pregnant women and it tends to appear earlier
than the other pregnancy-related dermatoses. Its incidence is not known but may
be as high as 1 in 5 to 1 in 20.
Risk factors
Risk factors for AEP include a
personal and /or family history of atopy.
Pathogenesis
AEP is thought to be triggered by pregnancy-‐specific shifts in cytokine profile expression leading to
preferential expression of T-helper 2 cytokines. To prevent fetal
rejection, a normal pregnancy is characterized by a lack of strong maternal
cell-mediated immune function and reduced Th1 cytokine production (e.g. IL-12,
interferon-γ) in contrast to the dominant
humoral immune response with increased Th2 cytokine production (e.g. IL-4,
IL-10). This natural switch towards a dominant Th2 response, which worsens the
imbalance already present in most atopic patients, is thought to favor the
development of AEP i.e. exacerbation of pre‐existing
atopic eczema and the first manifestation of atopic skin changes.
Clinical
features
Typical distribution of E-type AEP
In contrast to the
other specific dermatoses of pregnancy, AEP appears earlier, often during the
first trimester, with 75% of patients presenting before the third trimester. 20% patients suffer from an exacerbation of pre‐existing
atopic eczema with a typical clinical picture while remaining 80% experience
atopic skin changes for the first time. Of these, two‐thirds
present with widespread eczematous changes (so‐called E‐type
AEP) often affecting typical atopic sites such as the face, neck and flexural
surfaces of the limbs; one‐third of patients have papular
lesions (P‐type AEP) and would previously have been classified as prurigo of pregnancy.
Papular or P-type lesions are either scattered small
erythematous papules or
discrete, excoriated prurigo papules with a predilection for extensor surfaces
of the limbs, with truncal involvement less common as well as typical prurigo nodules, mostly located on the
shins and arms. Minor
features of eczema, including xerosis (often marked) or hyperlinear palms, may
be noted in patients with either subtype.
Disease
course and prognosis
Lesions respond quickly to therapy; however,
recurrence with subsequent pregnancies is common, consistent with an atopic
diathesis. Maternal and fetal prognoses are excellent, even in severe cases. In
a mother with a known history of atopy, the
infant may be at risk of developing atopic skin changes later on. In a mother with no prior history
of eczematous eruption, her risk of recurrence outside of pregnancy is unknown.
Diagnosis
The diagnosis is
largely clinical as histopathologic features are nonspecific. Direct and
indirect immunofluorescence
studies are negative. Total serum IgE is elevated in up to 70% of individuals
with AEP, usually to a mild degree. Serologic tests reveal no other
abnormalities.
Differential Diagnosis
Of the specific pregnancy dermatoses, PEP and
ICP are the ones that in particular need to be excluded. In AEP, the eruption
starts significantly earlier during gestation and has no association with
striae; serum bile acid levels are also normal. Furthermore, other pruritic
dermatoses not specifically associated with pregnancy (e.g. scabies, viral
exanthems, drug eruptions) must be considered.
Treatment
Cutaneous
lesions respond rapidly to mid potency
topical corticosteroids with or without systemic antihistamines. Emollients,
humectants, and topical antipruritic agents also play a role, as they do in
non-pregnant patients with atopic dermatitis. Topical urea (10%), polidocanol,
pramoxine, and menthol are considered safe during pregnancy. Phototherapy (UVB) is a safe additional tool, particularly
for severe cases in early pregnancy. Secondary bacterial infection may require
systemic antibiotics (e.g. penicillins, cephalosporins). Severe cases may require a short course of systemic
corticosteroids.
Treatment ladder
First line
·
Topical emollients
·
Topical corticosteroids
·
Oral antihistamines: loratadine and
cetirizine
Second line
·
Narrow‐band UVB
phototherapy
Third line
·
Prednisolone
Dermatoses
Associated with Fetal Risk in Pregnancy
At a Glance
|
·
Pemphigoid gestationis is an immunologically
mediated, intensely pruritic, vesiculobullous eruption of mid- to late
pregnancy that is associated with fetal risk. ·
Intrahepatic cholestasis of pregnancy represents
a reversible form of cholestasis in late pregnancy associated with
biochemical abnormalities and a risk of fetal complications, but invariably
lacking primary cutaneous lesions. Symptoms remit within 2–4 weeks of
delivery, but recurrences in subsequent pregnancies are common. ·
Pustular psoriasis of pregnancy is a rare, acute,
pustular eruption often accompanied by fever, leukocytosis, and an elevated
erythrocyte sedimentation rate. This is generally regarded as a variant of
psoriasis. |
Pemphigoid
gestationis (PG)
Salient features
·
Rare
pruritic vesiculobullous eruption that develops during
late pregnancy or the immediate postpartum period
·
Linear
C3 deposition along the basement membrane zone (BMZ) by direct IF
·
IgG1
autoantibodies directed against a transmembrane hemidesmosomal protein (BP180;
collagen XVII)
·
Increased
risk of prematurity and small-for-gestational age neonates; the risk correlates
with disease severity
·
Commonly
recurs in subsequent pregnancies
Introduction
Pemphigoid
gestationis (PG) is a rare, self-limited and intensely pruritic, autoimmune, bullous disorder that presents mainly in mid-to late
pregnancy or the immediate postpartum period. It is the most clearly characterized
dermatosis of pregnancy and the only one that may also affect the skin of the
newborn.
Epidemiology
The incidence of
pemphigoid gestationis has been estimated at 1: 2000 to 1: 50 000 pregnancies, depending on the prevalence of HLA-DR3 and -DR4 in different
populations. While occurring primarily during pregnancy and the immediate
postpartum period, pemphigoid gestationis has rarely developed in association
with trophoblastic tumors (hydatidiform mole, choriocarcinoma), implicating a
role for paternally derived tissues in the pathogenesis of this condition. Patients
with a history of pemphigoid gestationis appear to be at increased risk for the
development of Graves’s disease.
Pathophysiology
Historically, pemphigoid gestationis was
thought to be caused by an anti-BMZ “serum factor” (the “herpes gestationis
[HG] factor”) that induces C3 deposition along the dermal–epidermal junction.
This factor is now known to be complement-fixing autoantibodies of the IgG1
subclass directed against a 180 kDa transmembrane hemidesmosomal protein
(BPAG2; collagen XVII). As in patients with bullous pemphigoid (BP), it is the
non-collagenous (NC) segment closest to the plasma membrane of the basal
keratinocyte (NC16A) that constitutes the immunodominant region of BP180.
Circulating antibodies are almost exclusively directed against this domain, as
demonstrated by ELISA and immunoblot studies of maternal or neonatal sera.
Of interest, the
primary site of autoimmunity seems not to be the skin, but the placenta, as
antibodies bind not only to the basement membrane zone of the epidermis, but
also to the amniotic basement membrane (a structure
derived from fetal ectoderm and antigenically similar to skin). So attention has focused on immunogenetics and
potential cross-reactivity between placental tissue and skin. Women with
pemphigoid gestationis also have increased expression of MHC class II antigens
(DR, DP, DQ) within the villous stroma of their chorionic villi. It has
therefore been proposed that pemphigoid gestationis is a disease, initiated by
the aberrant expression of MHC class II antigens (of paternal haplotype), that
serves to initiate an allogeneic response to placental BMZ, which then
cross-reacts with skin.
Clinical
features
Pemphigoid gestationis presents with
intense pruritus that occasionally may precede skin lesions. There is
an abrupt onset of cutaneous lesions on the trunk, in particular the abdomen
and often within or immediately adjacent to the umbilicus. Rapid progression to
a generalized pemphigoid-like eruption then occurs, with pruritic urticarial
papules and plaques, followed by clustered (herpetiform) vesicles or tense
bullae on an erythematous base. The eruption may involve the entire body,
sparing only the mucous membranes. In
the pre‐bullous stage, differentiation between PG and PEP is almost
impossible, both clinically and histopathologically.
Disease
course and fetal prognosis
The
natural course of PG is characterized by exacerbations and remissions during
pregnancy, with spontaneous
improvement during late gestation is common. This is followed, however, by a
flare at the time of delivery in 75% of patients. Such flares may be dramatic,
with an explosive onset of blistering within hours. After delivery, the lesions usually resolve within weeks to
months.
Flares and/or recurrences in association with menstruation are common, and in
25–50% of patients, they may also be induced by oral contraceptives. Pemphigoid
gestationis may not develop during the patient’s first pregnancy, but,
once established, it is quite likely to recur in subsequent pregnancies,
usually with an earlier onset and more severe course. “Skipped” pregnancies
have been observed in 5–8% of women.
Approximately
10% of newborns develop mild skin involvement (neonatal
PG) due
to passive transfer of maternal antibodies and this resolves spontaneously
within days to weeks. There seems to be an increased risk of prematurity and
small-for-gestational age neonates, presumably due to chronic placental
insufficiency; the risk of these fetal complications correlates with maternal disease
severity (i.e. occurrence of blistering and early onset) and not with the use
of systemic corticosteroids. Therefore, women with PG should be followed
closely by their obstetrician. If the mother has received long term high doses
of prednisolone, the infant should be evaluated for evidence of adrenal
insufficiency.
Investigations
Patients in whom PG is suspected
usually require a biopsy for histopathology and DIF. Histopathologic findings
from lesional skin depend on the stage and severity of the disease. The pre‐bullous
stage is characterized by edema of the upper and middle dermis accompanied by a
predominantly perivascular inflammatory infiltrate, composed of lymphocytes,
histiocytes and a variable number of eosinophils. Histopathology of the bullous
stage demonstrates subepidermal blistering that, ultrastructurally, is located
at the lamina lucida of the dermo‐epidermal
junction.
Direct
immunofluorescence of perilesional skin, the gold standard in the diagnosis of
PG, shows linear C3 deposition along the dermo‐epidermal
junction in 100% of cases and additional IgG deposition in 30% of cases. Circulating
IgG antibodies in the patient's serum may be detected by indirect immunofluorescence
in 30–100% of cases, binding to the roof of the artificial split on salt‐split
skin. Determination
of antibody titers via BP180-NC16A ELISA may be helpful in following disease
activity and monitoring therapy.
Treatment
The
primary goal in treating this self-limited disease is to relieve pruritus and
suppress blister formation. In mild cases, the use of potent topical
corticosteroids combined with emollients and systemic antihistamines may be
adequate. However, systemic corticosteroids remain the cornerstone of therapy.
Most patients respond to 0.5 mg/kg of prednisolone daily; the dose is tapered
as soon as blister formation is suppressed. The common flare associated with
delivery usually requires a temporary increase in dosage. Persistent disease after delivery is uncommon and is treated like BP. Cases unresponsive to systemic corticosteroid treatment or
in cases where prolonged treatment with corticosteroid is contraindicated may
benefit from third line treatments including intravenous immunoglobulins and plasmapheresis
during pregnancy.
Treatment ladder
First line
·
Topical emollients: aqueous cream +
1–2% menthol
·
Potent topical steroids
·
Oral antihistamines: loratadine and
cetirizine
Second line
·
Prednisolone
Third line
·
Plasma exchange
·
Intravenous immunoglobulins
Cholestasis of pregnancy, intrahepatic
Salient features
·
Pruritus without primary skin lesions with an onset during the
third trimester
·
Secondary changes correlate with disease duration and vary from
subtle excoriations to severe prurigo nodularis
·
Elevated total serum bile acid levels are diagnostic; histology
is nonspecific and IF is negative
·
Increased risk of prematurity, intrapartum fetal distress, and
stillbirths
·
Recurs in 45–70% of subsequent pregnancies
Introduction
Intrahepatic cholestasis of pregnancy (ICP) is a rare,
reversible form of hormonally triggered cholestasis that typically develops in
genetically predisposed individuals in late pregnancy, when serum
concentrations of estrogen reach their peak.
Although maternal
prognosis is usually good (a small minority may develop steatorrhea and vitamin
K deficiency), fetal risk is significant. As a result, ICP is the most important
pruritic gestational condition to consider and promptly diagnose and treat in
order to prevent fetal impairment.
Epidemiology
It occurs in 1 in
1500 pregnancies and a positive family history is seen in 50% of affected
individuals. A higher incidence of ICP is also seen in multiple-gestation
pregnancies, which may be related to higher hormonal levels (e.g. estrogen) in
these patients.
Etiology and pathogenesis
The key
element is reduced excretion of bile acids, which leads to increased serum
levels. This not only provokes severe pruritus in the mother, but also may have
deleterious effects on the fetus. Toxic bile acids crossing the placenta can
lead to acute fetal anoxia due to abnormal uterine contractility and
vasoconstriction of chorionic veins as well as impaired fetal cardiomyocyte
function. One predisposing factor is mutations in genes (e.g. ABCB4) that encode bile transporter proteins. While
mild dysfunction of these canalicular transporters may not lead to clinical
symptoms in non-pregnant individuals, when the transporters’ capacity to
secrete substrates is exceeded (as occurs in the setting of high levels of sex
hormones during pregnancy), signs and symptoms of cholestasis can develop.
Other contributing factors are the cholestatic effect of estrogen and
progesterone metabolites, which peak late during pregnancy and hepatitis C
viral infection.
Although the
precise pathogenesis remains unclear, the interplay of hormonal, genetic,
environmental, and alimentary factors is thought to induce a biochemical
cholestasis in susceptible individuals. A prominent role for hormonal
alterations is suggested by the following observations: (1) ICP is a disease of
late pregnancy (corresponding to the period of highest placental hormone
levels); (2) ICP spontaneously remits at delivery when hormone concentrations
normalize; (3) twin and triplet pregnancies, characterized by greater rises in
hormone concentrations, have been linked to ICP; and, (4) ICP recurs during
subsequent pregnancies in an estimated 45%–70% of patients.
Geographic
variation and familial clustering indicate a genetic predisposition. ICP appears
to be a polygenetic condition. There are reports of higher incidence rates
during the winter months, and furthermore, dietary factors such as selenium
deficiency and increased intestinal permeability (“leaky gut“) have been
suggested as possible triggers, all point toward etiologic roles for
environmental and alimentary factors.
Clinical
features
ICP
is the only pregnancy dermatosis that presents without primary skin lesions. Patients typically
present during their last trimester with a sudden onset of intense, generalized
pruritus that often starts on the palms and soles. Pruritus begins during the first
and second trimester in 10% and 25% of cases, respectively. No primary skin
lesions are seen, and secondary changes due to scratching vary from subtle
excoriations early on to pronounced prurigo nodularis in those with pruritus of
longer duration. The extensor surfaces of the extremities, buttocks, and
abdomen are usually most severely affected. Initially, patients may complain of nocturnal
pruritus only, and symptoms generally are more severe at night throughout the
course of illness.
Constitutional
symptoms such as fatigue, nausea, vomiting, or anorexia may accompany the pruritus.
Progression to clinical jaundice, dark urine, or lightly colored stools occurs
in approximately one in five patients. Jaundice is usually a complication in those
with the most severe and prolonged episodes of ICP. In such patients,
concomitant extrahepatic cholestasis may be associated with steatorrhea and subsequent
vitamin K deficiency (malabsorption of fat-soluble vitamins including vitamin
K) leading to an increased risk of intra- and postpartum hemorrhage.
Disease
course and fetal prognosis
Pruritus typically
persists until delivery. The prognosis for the mother is
generally good as pruritus and associated biochemical abnormalities typically disappear spontaneously within 2 to 4 weeks after delivery. A protracted course is very unusual and
should prompt one to exclude other liver diseases, especially primary biliary
cirrhosis. Recurrences
during subsequent pregnancies occur in an estimated 45%–70% of patients. Some
women experience recurrent ICP after exposure to oral contraceptives or to
contraceptive aids, such as synthetic estrogens and progestational agents. In cases of jaundice and vitamin K deficiency, there is an
increased risk for intra‐ and postpartum hemorrhage in both the mother and child. Additionally,
affected women have a tendency toward the later development of cholelithiasis
or gallbladder disease.
ICP is
associated with significant fetal risk, in particular an increase in premature
births (20–60%), intrapartum fetal distress (20–30%; e.g. meconium staining of
amniotic fluid, abnormal fetal heart rate), and stillbirth (1–2%).
Fetal risk correlates with the elevation in serum bile acid levels, especially
when levels exceed 40 µmol/l. Such fetal
complications may be reduced with treatment and induction of labor after fetal
pulmonary maturation has been documented. Thus, prompt diagnosis and
treatment is essential, as is close obstetric surveillance.
Laboratory Studies
Histologic
findings in the skin and the liver are nonspecific and direct IF of
perilesional skin is negative. Elevation in serum
bile acid levels is the
single most sensitive indicator of ICP. The diagnosis is confirmed by an increase in
total serum bile acid levels (>11 µmol/l in a
pregnant woman that is
consistent with ICP. In healthy pregnant women, total bile acids (TBAs) are
slightly elevated above baseline and levels as high as 11.0 μM are accepted as
normal in late pregnancy. Clearly defined biochemical indices of ICP have not
yet been established. However, Brites et al identified the following common
features of ICP: (1) serum TBA concentrations greater than 11.0 μM (normal
range, 4.6–8.7 μM); (2) cholic acid–chenodeoxycholic acid ratio greater than
1.5 (normal range, 0.7–1.5) or cholic acid proportion of TBAs greater than 42%;
(3) glycine conjugates–taurine conjugates of bile acids ratio less than 1.0
(normal range, 0.9–2.0) or glycocholic acid concentration greater than 2.0 μM
(normal range, 0.6–1.5 μM). Degree of pruritus and disease severity generally
correlates with bile acid concentrations.
Mild perturbations in liver
function tests, including elevated transaminases, alkaline phosphatase,
5′-nucleotidase, cholesterol, triglycerides, phospholipids, and lipoprotein X
are commonly found. Among these parameters, alanine transaminase is
particularly sensitive, as an elevation in this enzyme is not a feature of
healthy pregnancies,
is usually elevated in those with ICP, but may be normal in 30% of patients. Gama glutamyl transferase, which
is generally low in late gestation, is typically normal or slightly elevated in
ICP. During
pregnancy, alkaline phosphatase levels typically increase (placental origin)
even in the absence of ICP. In
women with jaundice, conjugated (direct) bilirubin levels are increased and the
prothrombin time may be prolonged. Albumin may be slightly reduced, whereas α2-globulins
and β-globulins are appreciably elevated.
Hepatic
ultrasonography generally is normal.
Differential Diagnosis
Distinction
from other causes of pruritus in the pregnant woman can be challenging. The
presence of primary lesions points away from a diagnosis of ICP, which lacks
primary lesions. In
the absence of primary lesions, the clinical differential diagnosis includes
other causes of primary pruritus, including those that lead to cholestatic
pruritus. Viral hepatitis is a common disorder and should be excluded by
appropriate serologies. Of note, a history of hepatitis C viral infection is
considered a risk factor for the development of ICP, and in one study, 20% of
the women who were HCV RNA-positive developed ICP.
Other causes of liver derangement
and jaundice, such as non viral hepatitis, medications, hepatobiliary
obstruction, and other intrahepatic diseases (i.e., primary biliary cirrhosis)
must be ruled out. Finally, it must be remembered that hyperthyroidism,
allergic reactions, polycythemia vera, lymphoma, pediculosis, and scabies may
each manifest as generalized pruritus in pregnant as in nonpregnant women.
Treatment
Since
fetal prognosis correlates with disease severity, the therapeutic goal is
reduction of serum bile acid levels. This allows prolongation of the pregnancy
and lessens both fetal risk and maternal symptoms. An interdisciplinary approach
characterized by intense fetal surveillance is essential to the management of
ICP.
In mild cases, adequate relief of
maternal symptoms can be achieved with bland emollients and topical
antipruritic agents.
To date, the only
successful agent has been oral ursodeoxycholic acid (UDCA) as it reduces both maternal itch and fetal risk.
It
is a naturally occurring, hydrophilic, non-toxic bile acid that has been used
for a variety of cholestatic liver diseases. Although the exact mechanism of
action in ICP is still not fully understood, there is evidence that UDCA
corrects the maternal serum bile acid profile, decreases the passage of
maternal bile acids to the fetoplacental unit, and improves the function of the
bile acid transport system across the trophoblast. UDCA is safe for mother and
fetus, with its only side effect being mild diarrhea. Use of UDCA for ICP is
off-label as it is only approved for primary biliary cirrhosis. The recommended
oral dose is 15 mg/kg daily or, independent of body weight, 1 g daily, taken as
a single dose or divided into two to three doses. It should be started as early
as possible and administered until delivery. At this dose, UDCA is well tolerated and highly effective in
controlling the clinical and liver function abnormalities that define ICP.
In
jaundiced patients, the prothrombin time should be monitored, and intramuscular
vitamin K administered as necessary. In
addition to UDCA treatment, weekly fetal heart rate cardiotocographic
monitoring from 34 weeks' gestation onwards until childbirth. Early delivery (as
soon as fetal lung maturity is achieved at 36 to 37 weeks) is recommended by several authors.
Therapeutic ladder
First line
·
Topical emollients: aqueous cream +
1–2% menthol
·
Oral antihistamines: loratadine and
cetirizine
·
Oral UDCA 15 mg/kg/day
Second line
·
S‐adenosyl‐l‐methionine
·
Dexamethasone
·
Cholestyramine
Other recommendations
·
Weekly fetal cardiotocography to
monitor fetal heart rate and detect early signs of fetal distress
·
Maternal vitamin K replacement (if
jaundice is present)
·
Early delivery (36–37 weeks)
·
Dexamethasone may be needed for
fetal lung maturity
Pustular Psoriasis of Pregnancy (Impetigo Herpetiformis)
Introduction
Pustular psoriasis
of pregnancy is generally regarded as a variant of pustular psoriasis
attributable to hormonal alterations during pregnancy; however, some authors
maintain that it is a distinct clinical entity.
Von Hebra first
used the designation impetigo herpetiformis in 1872 to describe an acute
pustular eruption with usual onset during the third trimester of pregnancy.
Clinical Features
Pustular psoriasis of pregnancy
is characterized by an acute eruption occurring as early as the first, but
generally during the third, trimester of an otherwise uneventful pregnancy. The
condition manifests as erythematous patches whose margins are studded with subcorneal
pustules. The eruption typically originates in flexural areas but spreads
centrifugally and sometimes generalizes. Subungual lesions may result in
onycholysis. Rarely, mucous membrane involvement may lead to painful erosions.
The face, palms, and soles are commonly spared. The rash may be pruritic or
painful.
Onset of the eruption is
accompanied by such constitutional symptoms as fever, chills, malaise,
diarrhea, nausea, and arthralgias. Rarely, tetany, delirium, and convulsions
occur if hypocalcemia is severe.
Etiology and pathogenesis
Although generally regarded as a
form of pustular psoriasis, absence of a positive family history, abrupt
resolution of symptoms at delivery, and a tendency to only recur during
subsequent pregnancies distinguish this entity from generalized pustular
psoriasis. Moreover, factors known to trigger pustular psoriatic flares, such
as infection, exposure to culprit drugs, or abrupt discontinuation of systemic
corticosteroids are lacking in virtually all patients with pustular psoriasis
of pregnancy.
Diagnosis
Although the clinical picture is typical, a biopsy is helpful to confirm the diagnosis. Initial laboratory evaluation should include complete blood count and comprehensive metabolic panel with particular attention to calcium level.
Histopathologic examination
reveals classic features of pustular psoriasis.
The most common laboratory
derangements include leukocytosis, neutrophilia, an elevated erythrocyte
sedimentation rate, hypoferric anemia, and hypoalbuminemia. Less commonly,
calcium, phosphate, and vitamin D levels are decreased. Serum parathormone
levels are rarely decreased. Cultures of pustule contents and peripheral blood
are negative unless secondarily infected.
Clinical course and prognosis
Pustular psoriasis of pregnancy
classically presents during the last trimester, but there are reports of cases
occurring as early as the first trimester, during the puerperium, in
nonpregnant women taking oral contraceptives, and in postmenopausal women.
Symptoms are invariably progressive throughout pregnancy. A cardinal feature of
this disorder is the rapid resolution of symptoms after delivery. Recurrences
in subsequent pregnancies are common and characteristically are more severe
with onset earlier in gestation.
More widespread disease generally
portends a worse prognosis.
Complications
Life-threatening maternal
complications are infrequent today, but may result from profound hypocalcemia and bacterial sepsis. The most feared complications are placental insufficiency
and consequent stillbirth or neonatal death. For these reasons, early induction
of labor is often contemplated.
Treatment
Resolution after delivery is the
norm. However, given its consistently progressive course, treatment is
indicated to reduce the risk of fetal and maternal complications during
pregnancy. Topical treatments include wet dressings and topical corticosteroids,
but are rarely effective as monotherapy. Narrowband UVB combined with topical
steroids has been reported to be successful in rare cases as well.
Systemic corticosteroids were historically
the mainstay of therapy. Now cyclosporine and infliximab are deemed first line therapy. Cyclosporine has been successfully used at doses between 5 mg/kg
and 10 mg/kg daily. Infliximab, a TNF-α blocking agent has been successfully used
without adverse effect on the fetus, but with the caveat that live vaccines
should be delayed in newborns of mothers treated with infliximab. Although careful consideration of the benefits
and risks of TNF-blockade during pregnancy must be considered, these agents (including
etanercept and adalimumab) are Class B and may have a role in the management
of cases refractory to other therapies.
In all, cases, fluid status and
electrolytes should be monitored with rapid correction of imbalances. Fetal
monitoring is essential as decelerations in fetal heart rate may be the
earliest sign of fetal hypoxemia. Maternal cardiac and renal functions may be
compromised with disease progression and therefore should be monitored as well.
Induction of labor is an option when symptoms do not remit despite supportive
and pharmacologic therapy. The therapeutic armamentarium available after
pregnancy termination or after delivery in a non-nursing mother can be extended
to include oral psoralen and ultraviolet A (PUVA), oral retinoids, and methotrexate.
|
SPECIAL CONSIDERATIONS FOR CORTICOSTEROID AND
ANTIHISTAMINE USE DURING PREGNANCY |
|
|
Corticosteroids |
|
|
Topical |
Recent
large, population-based studies and a Cochrane review have not shown an increased risk of malformations,
including oral cleft palate, or preterm delivery Because
fetal growth restriction has been reported with extensive use of potent
corticosteroids (CS), particularly >300 g during the pregnancy, mild to
moderate CS are recommended over potent CS If
potent CS are required, the treatment period should be limited in duration Can
add to risk of developing striae |
|
Systemic |
Prednisolone
is the systemic corticosteroid of choice for dermatologic indications as it
is largely inactivated in the placenta (mother: fetus = 10: 1). The usual initial dose is 0.5–2 mg/kg/day depending
on the nature and severity of the disease. In treating pregnancy dermatoses,
corticosteroids are usually used only as a short‐term
therapy (<4 weeks) so that side effects are minimized. During
the first trimester, particularly between weeks 8 and 11, there is a possible
(debated) slightly increased risk of cleft lip/cleft palate, especially if
high doses prescribed (should not exceed
10–15 mg/day) and for >10 days; during this
same period, a longer duration of therapy appears safe if dosages are
<10–15 mg daily If
use is long-term and extends late into gestation, fetal growth should be
monitored and the risk of adrenal insufficiency in the newborn should be
addressed |
|
Antihistamines |
|
|
Systemic |
During
the first trimester, the classic sedating agents (e.g. chlorpheniramine,
diphenhydramine, clemastine, dimethindene) are preferred because of the
preponderance of safety data If
a non-sedating agent is requested, loratadine is the first choice and cetirizine
the second choice; both are considered safe throughout pregnancy, especially in the second and third trimester. |