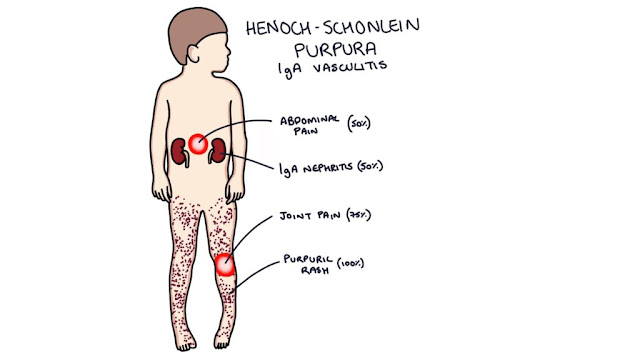
Henoch–schönlein purpura
Salient features
·
Most commonly occurs in children
<10 years of age and in association with a preceding respiratory infection,
but may also be seen in adults
·
Intermittent palpable purpura on
extensor extremities and buttocks
·
IgA-dominant immune deposits in
walls of small blood vessels
·
Arthralgias and arthritis
·
Abdominal pain and/or melena
·
Renal vasculitis often mild but can
be chronic
·
In adults, may be associated with an
underlying malignancy
Introduction
IgA
vasculitis, previously called Henoch–Schönlein purpura (HSP), is an immune
complex vasculitis,
a specific form of CSVV characterized by IgA1‐dominant immune deposits affecting small vessels
(predominantly capillaries, venules or arterioles) that typically
affects children following a recent
upper respiratory tract infection, especially with β-hemolytic streptococcus but
may also occur in adults. Sites of involvement include the skin, synovia,
gastrointestinal tract, and kidneys. The classic tetrad consists of palpable purpura (100%), arthritis (75%), abdominal pain (50%) and hematuria (50%).
Epidemiology
HSP is the most common form of vasculitis in children,
with an incidence of 10 to 20 cases per 100 000 children per year. The average
age of onset is 6 years and 90% of cases occur in children <10 years of age.
In adults, the incidence of HSP is 8 to 18 cases per million. HSP has a slight
male predominance in both children and adults.
Pathogenesis
HSP frequently presents 1 to 2 weeks following an upper
respiratory tract infection, especially in children. Streptococcal infections predispose
to IgA vasculitis, and antistreptolysin O titer positivity confers a 10‐fold risk of IgA vasculitis.
The pathogenesis of
IgAV is still largely unknown. The disease is characterized by IgA1-immune
deposits, complement factors and neutrophil
infiltration, which is accompanied with vascular
inflammation. Incidence of IgAV is twice as high during fall and winter,
suggesting an environmental trigger associated to climate. In IgA nephropathy immune complexes containing
galactose-deficient (Gd-)IgA1 are found and thought to play a role in
pathogenesis. Alternatively, it has been proposed that in IgAV IgA1 antibodies
are generated against endothelial cells and
that such IgA complexes can activate neutrophils via
the IgA Fc receptor FcαRI
(CD89), thereby inducing neutrophil migration and activation, which ultimately
causes tissue damage in IgAV.
Certain
genetic polymorphisms may predispose to more severe disease in HSP. For
example, HLA-B35 positivity may predispose to renal disease, while patients who
do not have the ICAM-1 469 K/E variant have less severe gastrointestinal
involvement.
IgA (specifically IgA1) is thought to play a significant
role in the pathogenesis of HSP, as IgA deposits in the walls of postcapillary venules
of the skin and in the renal mesangium, circulating immune complexes containing IgA and increased
serum levels of IgA (in 50% with active disease), have been demonstrated in
patients with HSP. In IgA vasculitis, IgA1 rather than IgA2 is the main IgA
subclass deposited in skin lesions. Lack
of glycosylation of the hinge region of IgA1 may promote the formation of macromolecular
complexes that lodge within the mesangium and activate the alternate complement
pathway.
Clinical features
Most
commonly, IgA vasculitis manifests at the outset with the classic findings of
purpura, arthralgia and abdominal pain. Fever occurs in approximately 20% of adults
and 40% of children. Rarely, gastrointestinal involvement
and arthritis can occur in the absence of skin disease.
The
cutaneous lesions begin as erythematous macules or urticarial papules, which may
evolve within 24 h into palpable purpura with hemorrhage. Hemorrhage vesicles and bullae and necrotic
ulcers may develop. A retiform pattern (raised, geometric, net like
presentation) within lesions is characteristic, but not always present. The
presentation may be identical to CSVV. Although it typically involves the
extensor aspects of the upper and lower limbs (especially the elbows and knees)
and buttocks in a symmetrical fashion, IgA vasculitis may also affect the trunk
and face. Individual
lesions usually regress within 10 to 14 days, with resolution of skin
involvement over a period of several weeks to months.
|
|
Extracutaneous manifestations of HSP are common. Painful
arthritis occurs in up to 75% of patients, most
frequently affecting the knees and ankles. Gastrointestinal involvement
(65% of patients) may precede the purpura and presents with bowel angina
(diffuse abdominal pain that is worse after meals), bowel ischemia, usually
including bloody diarrhea and/or vomiting. Intussusception and bowel
perforation are rare complications.
Renal
involvement with IgA vasculitis is common, occurring in approximately 40–50% of
patients; 25% have gross hematuria and the remainder microscopic hematuria.
Proteinuria occurs in 60% of these, but is uncommon in the absence of
hematuria. Although the appearance of cutaneous lesions often
precedes the development of nephritis, the latter is clinically evident within
3 months. In pediatric patients, risk factors for the development of nephritis
include age >8 years at onset, abdominal pain, and recurrent disease. Less common manifestations of IgA
vasculitis include orchitis (in 10–20% of boys), pancreatitis, neurological
abnormalities, uveitis, and carditis. The lung is also a rare site of involvement,
presenting as hemoptysis and/or pulmonary infiltrates due to diffuse alveolar
hemorrhage. Poor prognostic factors include renal failure at the time
of onset, nephrotic syndrome, and hypertension and decreased factor XIII
activity.
IgA small vessel vasculitis in adults, termed adult HSP,
should be considered separately, as the clinical presentation and prognosis
differ from that in children. For example, necrotic skin lesions are present in
60% of adults while cutaneous necrosis is observed in <5% of children.
Adults with IgA vasculitis are also more likely than children to develop
chronic renal insufficiency (up to 30%), especially if they have purpura above
the waist, fever and an elevated ESR. In addition, when CSVV is due to an
underlying neoplasm, the latter is usually a hematologic malignancy rather than a solid organ malignancy. However, 60–90% of adult patients with
neoplasm-associated IgA vasculitis will have cancer of a solid organ, in
particular lung. Adults are also more likely than children to have diarrhea and
leukocytosis, to require more aggressive therapy, and to have a longer hospital
stay.
Complications
and co‐morbidities
End‐stage renal disease is uncommon but, if it occurs, may need
renal transplantation. Renal transplant survival is over 80% at 5 years.
Disease
course and prognosis
- Abdominal
pain usually settles within a few days.
- About 25% of patients will
relapse
of the disease within 6 months
and typically the relapse is mild and easily treated.
- IgA vasculitis can become
chronic in 5–10% of patients, the cutaneous involvement usually lasting between
6 and 16 weeks.
- Patients
without kidney involvement can expect to fully recover within 4-6 weeks.
- Only 1% of these patients will go on to
develop end stage renal disease.
Investigations
IgA
vasculitis is a clinical diagnosis, with confirmation by direct
immunofluorescence and routine histology.
Leukocytoclastic vasculitis of
the small dermal blood vessels is seen. Perivascular
IgA deposits in DIF are characteristic of IgA
vasculitis and can help to distinguish it from other vasculitides including
CSVV. Of note, a small subset of patients has been described that meets
clinical criteria of HSP but lacks IgA deposition on DIF.
Differential diagnosis
Because up to 80% of all adults with CSVV may
demonstrate some vascular IgA deposition and IgA deposition can be seen in
other diseases (e.g. drug hypersensitivity, IgA monoclonal gammopathy,
inflammatory bowel disease, lupus erythematosus, cryoglobulinemia), a
diagnosis of HSP is supported by IgA predominance in
the correct clinical setting. Of the several proposals for diagnostic
criteria, the one developed by the European League against Rheumatism/Pediatric
Rheumatology European Society (EULAR/PReS), may be the most clinically relevant
to the dermatologist. In addition to palpable purpura (a required criterion),
at least one of the following must be present:
|
|
• |
|
|
|
• |
diffuse
abdominal pain that is worse after meals |
|
|
• |
any biopsy demonstrating predominant IgA deposition |
|
|
• |
Renal involvement in the form of hematuria and/or proteinuria. |
Treatment
Because
HSP is generally self-limited and resolves over the course of weeks to months,
treatment is mainly supportive. Dapsone and colchicine may decrease the
duration of cutaneous lesions and frequency of recurrences. Systemic
corticosteroids (prednisolone
1 mg/kg/day for 2 weeks, tapering over a further 2 weeks), are effective in treating the arthritis and abdominal pain
associated with HSP, as well as reducing the gastrointestinal complications and
duration of skin lesions, but do not prevent recurrences of purpura. Referral
to a nephrologist is appropriate for patients with evidence of renal
involvement. In adults, the following factors may predict relapsing disease:
age >30 years, an underlying systemic disorder, persistent purpura >1
month, abdominal pain, hematuria, and absence of IgM on DIF.
Considerable controversy
surrounds the use of corticosteroids and/or immunosuppressive medications for
the treatment of severe renal disease and for preventing renal sequelae in
individuals who have severe renal involvement. Pulsed intravenous methylprednisolone,
cyclosporine A, cyclophosphamide, azathioprine and mycophenolate mofetil are used
for the treatment of severe renal disease. In sum, the current consensus
appears to be that corticosteroids do not prevent renal disease but could be
used to treat severe nephritis.